Obstetrical and neonatal outcomes of cardio-facio-cutaneous syndrome: Prenatal consequences of Ras/MAPK dysregulation
Funding information: National Institutes of Health; NIH/NIDDK, Grant/Award Number: 5K23DK119949-04; NIH/NICHD, Grant/Award Numbers: R01HD107190, 5K12HD001262-18; Chan Zuckerberg Biohub; Brianna Marie Foundation; Doris Duke Charitable Foundation
Abstract
We systematically delineated the prenatal phenotype, and obstetrical and neonatal outcomes of the RASopathy cardio-facio-cutaneous (CFC) syndrome. A comprehensive, retrospective medical history survey was distributed to parents of children with confirmed CFC in collaboration with CFC International, Inc. Data were collected on CFC gene variant, maternal characteristics, pregnancy course, delivery, and neonatal outcomes with the support of medical records. We identified 43 individuals with pathogenic variants in BRAF (81%), MEK1 (14%), or MEK2 (5%) genes. The median age was 8.5 years. Hyperemesis gravidarum, gestational diabetes, gestational hypertension, and preeclampsia occurred in 5/43 (12%), 4/43 (9%), 3/43 (7%), and 3/43 (7%) of pregnancies, respectively. Second and third trimester ultrasound abnormalities included polyhydramnios, macrocephaly, macrosomia, and renal and cardiac abnormalities. Delivery occurred via spontaneous vaginal, operative vaginal, or cesarean delivery in 15/42 (36%), 7/42 (16%), and 20/42 (48%), respectively. Median gestational age at delivery was 37 weeks and median birth weight was 3501 grams. Germline pathogenic vaiants had mutiple congenital consequences including polyhydramnios, renal and cardiac abnormalities, macrosomia, and macrocephaly on second and third trimester ultrasound. Elevated rates of operative delivery and neonatal complications were also noted. Understanding and defining a prenatal phenotype may improve prenatal prognostic counseling and outcomes.
1 INTRODUCTION
Cardio-facio-cutaneous (CFC) syndrome is one in a group of disorders, collectively termed RASopathies, that arise from genetic variants in components that encode the critical Ras/mitogen-activated protein kinase (RAS/MAPK) signaling pathway (Rauen, 2013, 2022). In addition to CFC, the RASopathies include neurofibromatosis type 1, Noonan syndrome (NS), NS with multiple lentigines, Costello syndrome (CS), Legius syndrome, capillary malformation-arteriovenous malformation syndrome, and SYNGAP1 autism. Together, the RASopathies are considered one of the most common groups of congenital anomaly syndromes.
CFC syndrome is due to heterozygous germline pathogenic variants in protein kinase genes BRAF (Niihori et al., 2006; Rodriguez-Viciana et al., 2006), MEK1/MAP2K1 (Rodriguez-Viciana et al., 2006), or MEK2/MAP2K2 (Rodriguez-Viciana et al., 2006), and rarely in KRAS, a small GTPase (Niihori et al., 2006). Heterozygous activating variants in the BRAF gene account for the majority of CFC cases, with MEK1 and MEK2 variants being the next most prevalent (Tidyman & Rauen, 2010). Disease-causing variants for CFC syndrome are usually de novo, although there have been rare reported cases of autosomal dominant inheritance (Karaer et al., 2015; Linden & Price, 2011; Rauen, 2010; Rauen et al., 2010). The postnatal clinical features of CFC are heterogenous and can vary between affected individuals. Distinctive craniofacial dysmorphism may include relative macrocephaly, hypoplasia of the supraorbital ridges, hypertelorism, down-slanting palpebral fissures, short nose with depressed bridge and anteverted nares, low-set ears that may be posteriorly rotated, deep philtrum, and relative micrognathia. Congenital cardiac abnormalities are commonly associated with CFC syndrome, particularly pulmonary valve stenosis, septal defects, and hypertrophic cardiomyopathy. Cutaneous abnormalities are also a major feature of CFC syndrome, including hyperkeratosis, ichthyosis, pigmented moles, and abnormal hair. Hypotonia, speech delay, and moderate-to-severe intellectual delay are almost always present in affected individuals.
Many of these postnatal features can be visualized by prenatal ultrasound (Stark et al., 2012), while others such as intellectual delay are not possible to detect prior to birth. Additionally, some features of RASopathies such as nonimmune hydrops fetalis are unique to the prenatal setting. As next generation sequencing (NGS), including exome sequencing, has become more accessible, prenatal diagnosis of RASopathies has become more common (Sparks et al., 2020). Therefore, studies are underway to identify a prenatal phenotype to alert the physician to a possible diagnosis of a RASopathy, and guide subsequent genetic analysis and prenatal outcomes. These findings include nonimmune hydrops fetalis, polyhydramnios, increased nuchal translucency (NT) or cystic hygroma, macrosomia, macrocephaly, cardiac and renal anomalies, and lymphatic abnormalities (Myers et al., 2014; Sparks et al., 2020). Unfortunately, none of the above ultrasound findings are specific for CFC syndrome, with many of the phenotypic features being shared with other RASopathies as well as other categories of genetic disorders. For example, a thickened NT is associated with all RASopathies (Ali et al., 2017) which should be considered if an increased NT is noted in a euploid fetus.
Another consequence of the overlapping phenotype is that in the absence of wide utilization of prenatal exome sequencing, diagnosis is often delayed to the neonatal period, when molecular analysis is performed for dysmorphia. Therefore, much of what is known about the natural history of CFC syndrome pertains to the postnatal phenotype. While several studies (Biard et al., 2019; Mucciolo et al., 2016; Myers et al., 2014; Scott et al., 2021; Sparks et al., 2020; Stuurman et al., 2019) have described prenatal features of the RASopathies in general, there remains a paucity of data pertaining to the prenatal features of CFC in particular. Thus, we sought to delineate the prenatal phenotype, and the obstetrical and neonatal outcomes, of pregnancies after which the child was confirmed to have CFC syndrome. This large retrospective cohort study describes the prenatal features and perinatal complications associated with variant-confirmed CFC syndrome, which is important for guiding accurate prenatal diagnosis, anticipation of perinatal complications, and focused postnatal medical care.
2 MATERIALS AND METHODS
A comprehensive questionnaire was distributed to parents by CFC International, Inc. (www.cfcsyndrome.org) investigating the antepartum, intrapartum, and postpartum periods of pregnancies in which the fetus, now child, was ultimately found to harbor a pathogenic variant postnatally for CFC syndrome. CFC International is a support group for families with the mission to promote research and educate its members, the public, and the medical community. Data were collected on CFC gene and variant, maternal characteristics, pregnancy complications, delivery outcomes, and neonatal outcomes. Maternal characteristics included past medical history, medications, and parental exposures. Pregnancy outcomes included detailed prenatal care and testing stratified by trimester. Delivery outcomes included gestational age, delivery method, complications, anthropometric measurements, and Apgar scores. Neonatal outcomes identified included need for hospitalization, respiratory issues, serum abnormalities, and feeding difficulties.
When available, medical records were reviewed. Results were computed based upon respondents with completed answers, with blank entries excluded from the denominator. Only individuals confirmed to possess known pathogenic variants for CFC syndrome were included in the study. Therefore, any participant with a clinical diagnosis but absence of genetic confirmation was excluded. Summary statistics were computed using Microsoft Excel (Microsoft Corporation, Redmond, WA). This study was approved by the Committee on Human Research at the University of California and approved by CFC International, Inc. All participants provided written informed consent.
3 RESULTS
3.1 General demographics
Of the 47 surveys completed, 4 were excluded due to lack of a specific pathogenic variant identified. All 43 included participants (26 females and 17 males) had a confirmed disease-causing pathogenic variant. The most common gene mutation identified was BRAF in 81% (n = 35) of the study population, and MEK1 (n = 6) and MEK2 (n = 2), with 14% and 5%, respectively. The average age of the cohort was 8.5 years, with a range of 3 months to 22.3 years of age. The majority of the cohort identified as Caucasian (88%), but also included were Latino (9%) and Asian (3%) individuals. At the time of delivery, the median maternal age was 30 years (21–43 years range) and paternal age was 32 years (20–52 years range). The majority of the participants were multiparous, with an average of three prior pregnancies. Of the total 43 parents, 41 reported conceiving spontaneously, one participant conceived by in vitro fertilization and one participant conceived following ovulation induction.
3.2 Pregestational and intrapartum characteristics
None of the study participants reported a history of pregestational diabetes or hypertension. However, four (9%) were diagnosed with gestational diabetes and six (14%) were diagnosed with gestational hypertension (Table 1). The diagnosis of gestational diabetes is similar to 7% reported in the general population (Etminan-Bakhsh et al., 2020), while the diagnosis of gestational hypertension is nearly double the 7% general population prevalence (Sadanandan et al., 2019). Intrapartum infections affected 14% of the study population which is similar to the baseline risk of 10% (Hastings-Tolsma et al., 2013). Notably, 12% of the study population reported receiving a diagnosis of hyperemesis gravidarum in early pregnancy, significantly higher than the 0.3%–3% incidence in the general population (Bulletins-Obstetrics CoP, 2018).
| Pregnancy comorbidities/complications | Number affected | Percent of total (n = 43) | Percent of general population affected |
|---|---|---|---|
| Gestational diabetes | 4 | 9.3% | 6% |
| Pregnancy-induced hypertensiona | 6 | 14% | 5% |
| Hypothyroidism | 2 | 4.7% | 2% |
| Infectionb | 6 | 14.2% | 3% |
| Hyperemesis gravidarum | 5 | 11.6% | 3% |
| Asthma | 1 | 2.3% | 5% |
- a Pregnancy-induced hypertension includes those diagnosed with gestational hypertension, preeclampsia with or without severe features, as defined by American College of Obstetricians and Gynecologists (Bulletins—Obstetrics ACoOaGCoP, 2020).
- b Antepartum infections reported include recurrent urinary tract infections, sinus infection requiring antibiotics, and varicella (chicken pox).
3.3 Prenatal screening and testing
All study participants reported engagement in some form of prenatal screening (Table 2). First trimester ultrasounds were performed in 31 (72%) participants, of which only 2 were abnormal and notable for cystic hygromas. First trimester biochemistry maternal serum screening, which measures serum pregnancy-associated plasma protein A and free beta-human chorionic gonadotropin (hCG) at 10–14 weeks of gestation to determine the risk of fetal Trisomy 21 and Trisomy 18, was performed in one study participant, and was normal.
| Timing | Study | Total number of those who responded n = 43 (%) | Abnormal (%) |
|---|---|---|---|
| First trimester | |||
| First trimester ultrasounda | 31 (72.1) | 3 (9.6) | |
| First trimester biochemistry screenb | 1 (2.3) | 0 | |
| Chorionic villus sampling | 2 (4.7) | 0 | |
| Second trimester | |||
| Triple screenc | 19 (44.2) | 3 (15.8) | |
| Quadruple screend | 1 (2.3) | 0 | |
| Second trimester ultrasounde| | 42 (97.7) | 25 (59.5) | |
| 3D/4D | 6 (14.0) | 3 (50.0) | |
| Fetal echocardiogram | 9 (20.9) | 3 (33.3) | |
| Amniocentesis | 14 (32.6) | 0 | |
| Third trimester | |||
| Third trimester ultrasound | 28 (65.1) | 22 (78.6) | |
| 3D/4D | 5 (12.0) | 0 | |
- a First trimester ultrasound includes any ultrasound performed from estimated date of confinement (EDC) to 12 weeks of gestation.
- b First trimester biochemistry screen: Performed between 11 and 14 weeks gestation and measures maternal serum free beta-human chorionic gonadotropin, pregnancy-associated plasma protein A, providing a risk assessment for trisomy.
- c Triple screen: maternal serum screen measuring alpha-fetoprotein, total beta-human chorionic gonadotropin, and unconjugated estriol to determine risk of chromosomal abnormality or open neural tube defect in the infant.
- d Quadruple screen: maternal serum screen measuring alpha-fetoprotein, total beta-human chorionic gonadotropin, unconjugated estriol, and inhibin A to determine risk of fetal chromosomal abnormality or open neural tube defect.
- e Standard 2D anatomy ultrasound performed between 15 and 20 weeks gestational age.
Sixteen study participants, or 37%, reported undergoing invasive prenatal diagnosis, either chorionic villus sampling (CVS) or amniocentesis depending on gestational age. Specifically, two underwent CVS, and 14 underwent amniocentesis. None of these individuals reported abnormal results as testing at that time was limited to fluorescence in situ hybridization and karyotype for aneuploidy (Table 2).
In the second trimester, 19 participants reported having a triple maternal serum screen performed (Table 2). Two individuals reported an abnormal alpha fetoprotein, one reported an abnormal total beta-hCG, and none reported abnormal estriol levels. Of the three participants that reported abnormal triple screens, two reported the result of “high risk for trisomy 21” and one reported “other” but did not further elaborate on a specific diagnosis.
Total 42 participants (98%) had a second trimester ultrasound of which 60% were abnormal (Table 3 and Figure 1). The most prevalent second trimester ultrasound abnormalities were polyhydramnios (52.4%), genitourinary system anomaly (31%), macrocephaly (19%), and size greater than dates (19%). Additionally, 11.9% of the study population reported that the second trimester ultrasound demonstrated a thickened nuchal fold or cystic hygroma (Table 3). These findings were again noted in the third trimester (Table 3).
| Timing | Organ system | n (%) | Abnormalitya | n (%) |
|---|---|---|---|---|
| First trimester | n = 31 | n = 31 | ||
| Nuchal translucency | 2 (12.9) | Cystic hygroma | 2 (12.9) | |
| Second trimester | n = 42 | n = 42 | ||
| Placenta/cord/fluid | 24 (57.1) | Polyhydramnios Placentomegaly Single umbilical artery |
22 (52.4) 1 (2.4) 1 (2.4) |
|
| Growth | 10 (23.8) | Macrosomia Fetal growth restriction |
8 (19.0) 2 (4.8) |
|
| Renal | 9 (21.4) | Hydronephrosis Hydroureter |
8 (19.0) 1 (2.4) |
|
| Cardiac anomaly | 6 (14.2) | Cardiomegaly Transposition of the great vessels Pericardial effusion Valvular abnormalityb Unspecified cardiac anomaly |
2 (4.8) 1 (4.2) 1 (2.4) 1 (2.4) 3 (2.4) |
|
| Cerebral | 11 (26.2) | Macrocephaly Ventriculomegaly Mega cisterna magna |
9 (21.4) 2 (4.8) 1 (2.4) |
|
| Neck | 5 (11.9) | Cystic hygroma Increased nuchal fold |
4 (9.5) 1 (2.4) |
|
| Gastrointestinal | 3 (7.2) | Tracheo-esophageal fistula Small stomach Large stomach |
1 (2.4) 1 (2.4) 1 (2.4) |
|
| Skeletal | 3 (7.2) | Short femur Abnormal spine |
2 (4.8) 1 (2.4) |
|
| Third trimester | n = 28 | n = 28 | ||
| Placenta/cord/fluid | 17 (60.7) | Polyhydramnios Placentomegaly Single umbilical artery |
15 (53.6) 1 (3.5) 2 (7.1) |
|
| Growth | 6 (21.4) | Macrosomia Fetal growth restriction |
4 (14.3) 2 (7.1) |
|
| Renal | 5 (17.8) | Hydronephrosis |
5 (17.8) | |
| Cardiac anomaly | 5 (17.8) | Transposition of great vessels Tetralogy of Fallot Coarctation of the aorta Atrial septal defect |
1 (3.5) 1 (3.5) 1 (3.5) 1 (3.5) |
|
| Cerebral | 5 (17.8) | Ventriculomegaly Dandy walker |
2 (7.1) 1 (3.5) |
|
| Neck | 4 (14.2) | Nuchal thickening Cystic hygroma |
1 (3.5) 1 (3.5) |
|
| Gastrointestinal | 3 (10.7) | Tracheo-esophageal fistula Small stomach Large stomach |
1 (3.5) 2 (7.1) 1 (3.5) |
|
| Skeletal | 3 (10.7) | Short femur Abnormal spine (hemivertebrae) |
1 (3.5) 1 (3.5) |
|
- a Each abnormality is independently tabulated.
- b Probands may have one ore multiple abnormalities. Each abnormality is independently tabulated.
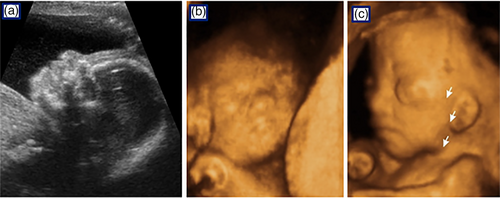
Six participants (16.7%) reported that the second trimester ultrasound revealed a cardiac anomaly. One case was prenatally diagnosed as transposition of the great vessels, two cases had cardiomegaly including one with tricuspid and mitral valve regurgitation concerning for right heart failure, three additional cases had an unspecified cardiac anomaly including one with a pleural effusion. Fetal echocardiograms were performed on nine study participants, and of these 33% were abnormal and confirmed the second trimester findings.
A third trimester ultrasound was performed in 65% of study participants. Of these, 79% (22/28) were abnormal, suggesting that as gestational age increases, detection of fetal anomalies similarly increases (Table 3). The most frequently reported indications for a third trimester ultrasound were decreased fetal movement and polyhydramnios. Polyhydramnios was noted in 54% (15/28) of the study population in the third trimester. Total 18% (5/28) of study participants reported a finding of hydronephrosis, and 14% (4/28) reported that fetal size was greater than expected for gestational age, referred to as “size greater than dates” (Table 3).
Of note, although the survey specifically inquired about facial abnormalities, none were reported in our study population. Eight study participants (19%) did endorse a finding of macrocephaly in the second trimester. Two participants reported ventriculomegaly in the second trimester, and one reported a finding of a large posterior fossa. While the individual who was noted to have a large posterior fossa did not report this finding in the third trimester, those that reported ventriculomegaly in the second trimester reported the same finding in the third trimester (Table 3). A total of six participants underwent a 3D/4D ultrasound in the second trimester (Table 2), and only five underwent the same study in the third trimester (Table 2). No participants reported undergoing a fetal magenetic resonance imaging during their prenatal course.
About 25% (11/43) of participants reported that they were counseled that the fetus likely had a “genetic syndrome,” but no specific diagnosis could be made prenatally. Six (14%) of the participants were given a differential diagnosis that included Trisomy 21. Additional differential diagnoses included Turner syndrome, CS, NS, Beckwith-Wiedemann syndrome, unspecified metabolic disorder, and Dandy Walker syndrome.
3.4 Obstetrical outcomes
Almost half of the study participants were delivered at a premature gestational age, with an average gestational age of 35 weeks and 6 days (±3 weeks). About 19% of study participants delivered at less than 34 weeks' gestation and 30% delivered in the late preterm period. This included 14% of participants who reported experiencing preterm labor, and 7% with preterm premature rupture of membranes (Table 4). Only 12% of study participants delivered at a gestational age of 39 weeks or greater (Table 4).
| Outcome | n = 43 (%) | |||
|---|---|---|---|---|
| Preterm premature rupture of membranes | 3 (7.0) | |||
| Preterm labor | 6 (14.0) | |||
| Meconium-stained fluid | 7 (16.3) | |||
| Average gestational age at birth | 35 weeks 6 days ±3 weeks |
|||
| Gestational age of delivery | n = 43 (%) | n = 43 (%) | ||
| Term | 22 (51.1) | >39 weeks | 5 (11.6) | |
Preterm |
21 (48.9) |
37 weeks to 38 weeks 6 days 34 weeks to 36 weeks 6 days <34 weeks |
17 (39.5) 13 (30.2) 8 (18.6) |
|
| Mode of delivery | n = 42 (%)a | n = 42 (%) | ||
Vaginal delivery |
22 (52.4) | Spontaneous Forceps-assisted Vacuum-assisted |
15 (35.7) 4 (18.2) 3 (13.6) |
|
| Cesarean delivery | 20 (47.6) | Repeat elective Malpresentation Placenta previa Fetal intolerance of labor Macrosomia Arrest of dilation or descent Failed vacuum-assisted vaginal delivery |
6 (30) 3 (15) 1 (5) 6 (30) 1 (5) 2 (10) 1 (5) |
|
- a One respondent did not complete mode of delivery section of survey, therefore total number of respondents is 42 (n = 42).
Of the individuals who reported having a vaginal delivery, 68% had spontaneous onset of labor. About 14% of vaginal deliveries were vacuum-assisted and 18% were forceps-assisted (Table 4). The indication for operative delivery was not part of the scope of the survey. Notably, almost one half of the study population underwent cesarean delivery (Table 4), which is higher than the estimated 30% rate in the general obstetric population (Clapp & Barth, 2017). Of the individuals who reported a cesarean delivery, half were planned, or scheduled, prior to delivery. About 30% of study participants elected for a repeat cesarean delivery, and 15% underwent a cesarean delivery for malpresentation (Table 4). Of those who did not have a planned cesarean delivery, the indication for cesarean delivery was fetal intolerance of labor in 30% and arrest of labor in 10% (Table 4).
3.5 Neonatal outcomes
Pathogenic variant confirmed CFC-affected infants had an average Apgar score of 6 and 7 at 1 and 5 min, respectively (Table 5). Of the 23 infants for whom 5-min Apgar scores were available, 6 (26%) were less than 7. The average head circumference in our study population was 34.3 ± 3.2 cm, and the average birth weight was 3233 ± 890.4 g.
| Neonatal characteristics | Number of respondents | Study population characteristics |
|---|---|---|
Average weight in grams Average head circumference in cm Average Apgar score at 1 min Average Apgar score at 5 min |
42 18 24 23 |
3257.1 ± 843.9 g 34.5 ± 3.0 cm 6.2 ± 2.3 7.7 ± 1.7 |
| Neonatal complications | Number affected | Percent of respondents |
Irregular heart beat Feeding tube requirement Edema (including nuchal edema) Chylothorax Need for intubationa Hyperbilirubinemia Hypoglycemia Average length of hospitalizationb |
9/41 24/42 17/41 4/41 11/43 10/42 4/39 42 |
22% 57% 41% 10% 26% 24% 10% 35.9 days |
- a Length of intubation ranged from 0.5 to 42 days.
- b Range for length of hospitalization was 1–111 days.
The majority of CFC infants had a complicated course during the birth hospitalization, with an average length of hospitalization of 35.9 days and ranging from 1 to 111 days (Table 5). Grossly apparent edema was reported in 41% (17/41) of the participants. The majority, 57% (24/42) of CFC-affected infants required a feeding tube for poor oral intake, likely in part due to associated hypotonia (Table 5).
Respiratory issues were common in the study population. About 26% (11/43) of the infants required intubation with a mean duration of 14 days. One other infant required continuous positive airway pressure for respiratory support. About 10% (4/41) of the study population reported receiving a diagnosis of chylothorax. Cardiovascular issues were also prevalent among CFC-affected infants, and an arrhythmia was noted in 22% (9/41) (Table 5). Other infant complications included hyperbilirubinemia, with 24% (10/42) participants requiring use of lights for treatment of hyperbilirubinemia. Additionally, 24% of infants received a course of intravenous antibiotics for rule-out sepsis.
4 DISCUSSION
We present a large cohort of CFC cases with prenatal features that provides generalized information about the prenatal phenotype in cases with confirmed molecular diagnosis. The detailed information will be invaluable to prenatal geneticists who correlate prenatal diagnosis with sonographic fetal findings. The data provide targeted phenotypic features that could improve the accuracy of prenatal diagnosis using gene panels and exome sequencing, as well as inform families and providers about expected perinatal outcomes.
In the era of NGS, there is increasing interest in defining phenotypic features that would guide clinicians to recommend prenatal RASopathy testing. RASopathies are increasingly being diagnosed in utero (Stuurman et al., 2019). Stuurman et al. recently published a retrospective study on the prenatal findings associated with mutation confirmed NS, a RASopathy that affects 1 in 1000 to 2500 live births (Nora & Fraser, 1981). This study revealed that all but one case of prenatally diagnosed, mutation-confirmed NS, had a thickened NT. Therefore, the authors recommend NGS testing for RASopathies when the NT is ≥5 mm, and also recommend testing if the NT is ≥3.5 mm and at least one other anomaly is present on prenatal ultrasound (Stuurman et al., 2019). It appears that this finding differs significantly by cases diagnosed prenatally versus postnatally as the reported increased NT rate in our cohort was only 12.9%.
While the prenatal phenotype is rapidly evolving, the neonatal phenotype of CFC, affecting 1/810,000 births (Abe et al., 2012), is well described by characteristic features including macrocephaly with a large forehead, bi-temporal narrowing, increased facial width, and coarse facies, sparse or absent hair and eyebrows, and down-slanting palpebral fissures and epicanthic folds. Additionally, neonates will often have low-set, posteriorly rotated ears. This dysmorphology is often challenging to adequately detect sonographically prenatally, however other features such as congenital heart defects, diagnosed in 45% of those with CFC syndrome, are expected to be visualized. Our cohort is larger than a previously presented cohort by Templin et al. (2016). A recent cohort by Scott et al. (2021) presents data from a large, multicenter cohort with similar prenatal finding. We only detected a cardiac anomaly in 17.8% of cases. We are, as suggested by these other prenatal reports, reliant on other sonographic features such as a thickened NT or cystic hygroma, or polyhydramnios to aid with the diagnosis. The data presented in the present study also suggest that CFC should be suspected in cases of fetal macrosomia with short femurs, hydrops fetalis, fetal hydronephrosis, ventriculomegaly, and fetal macrocephaly. Evidence of more than one of these abnormalities should prompt genetic counseling and the option for definitive genetic diagnosis.
Our study is unique to prior studies in that obstetric and neonatal outcomes were documented. The data reveal that mutation-positive CFC syndrome is associated with an increased rate of preterm delivery, cesarean delivery, and operative vaginal birth. The increased rate of preterm labor and premature preterm rupture of membranes, may be associated with the almost universal prevalence of polyhydramnios in our study population. Polyhydramnios causes uterine over-distention and subsequent inflammatory cytokine release that can induce preterm labor and preterm premature rupture of membranes (Adams Waldorf et al., 2015). The data also suggest that pregnancies affected by CFC syndrome have a higher risk of operative vaginal delivery and cesarean delivery. While the indications for each operative-assisted vaginal and cesarean delivery were not specifically asked in our survey, the fact that 41% of all vaginal deliveries were operative, suggests that there are specific characteristics of CFC-affected neonates that warrant this intervention. Specifically, one could surmise an increased risk of fetal distress as well as labor arrest associated with the findings of fetal macrosomia and macrocephaly in CFC-affected individuals.
Neonatal outcomes of CFC individuals were significant, but were confounded by the rate of prematurity. About 26% of study participants reported a 5-min Apgar score of less than 7, suggesting that these neonates may require more intensive resuscitation immediately following delivery. This is further evident by the high rate of neonatal intubation in our study population at 26%. Neonatal hypotonia likely contributed to the need for respiratory support, which is further supported by the fact that over 50% of our study population required enteral feeding for nutritional support postnatally.
There are several limitations of this study. Recall bias is inherent in the retrospective design. Attempts were made to minimize this bias by review of the medical record when available. Our results are limited by the number of responders as well as the wide range of birth dates which could potentially impact the prenatal data available for those who responded. Furthermore, while attempts were made to include a diverse population of affected individuals, our questionnaire was distributed through CFC International, therefore limiting our sample to those individuals involved in the organization. In addition, the high frequency of prematurity may be a confounder that increased the risk of a neonatal complication including difficulties feeding and the need for ventilatory support.
In conclusion, this retrospective cohort study provides further evidence and characterization of the fetal phenotypic features associated with CFC syndrome. With the subsequent improvement and increased utilization of 3D ultrasound technology, the data presented herein aim to assist obstetricians and genetic counselors in providing anticipatory guidance with regard to a possible diagnosis of CFC syndrome. Importantly and unique to our study, the data also highlight and quantify the increased risk of adverse obstetric and neonatal outcomes in this population. Further investigation into the indications for obstetric intervention and the maternal and neonatal outcomes associated with the various modes of delivery in this population may provide further guidance on optimal intrapartum management of these patients as well as the neonatal risks postpartum.
ACKNOWLEDGMENTS
The authors wish to acknowledge the support and dedication of Molly Santa Cruz and Brenda Conger of CFC International, and to all the CFC families who contributed to this study.
FUNDING INFORMATION
Dr. Jelin is funded by the NIH/NIDDK (5K23DK119949-04). Dr. Sparks is funded by grants from the NIH/NICHD (5K12HD001262-18, R01HD107190), the Chan Zuckerberg Biohub, the Brianna Marie Foundation in collaboration with the Fetal Health Foundation, and the Doris Duke Charitable Foundation. The funding sources had no role in the data collection, writing of the report, or in the decision to submit the article for publication.
CONFLICT OF INTEREST
The authors report no disclosures.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




