A homozygous missense variant in the MLC1 gene underlies megalencephalic leukoencephalopathy with subcortical cysts in large kindred: Heterozygous carriers show seizure and mild motor function deterioration
Funding information: Deanship of Scientific Research Taibah University, Grant/Award Number: 10057
Abstract
Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is a rare type of leukodystrophy characterized by epileptic seizures, macrocephaly, and vacuolization of myelin and astrocyte. The magnetic resonance imaging of the brain of MLC patients shows diffuse white-matter anomalies and the occurrence of subcortical cysts. MLC features have been observed in individuals having mutations in the MLC1 or HEPACAM genes. In this study, we recruited a six generation large kindred with five affected individuals manifesting clinical features of epileptic seizures, macrocephaly, ataxia, and spasticity. In order to identify the underlying genetic cause of the clinical features, we performed whole-genome genotyping using Illumina microarray followed by detection of loss of heterozygosity (LOHs) regions. One affected individual was exome sequenced as well. Homozygosity mapping detected several LOH regions due to extensive consanguinity. An unbiased and hypothesis-free exome data analysis identified a homozygous missense variant (NM_015166.3:c.278C>T) in the exon 4 of the MLC1 gene. The variant is present in the LOH region on chromosome 22q (50 Mb) and segregates perfectly with the disorder within the family in an autosomal recessive manner. The variant is present in a highly conserved first cytoplasmic domain of the MLC1 protein (NM_015166.3:p.(Ser93Leu)). Interestingly, heterozygous individuals show seizure and mild motor function deterioration. We propose that the heterozygous variant in MLC1 might disrupt the functional interaction of MLC1 with GlialCAM resulting in mild clinical features in carriers of the variant.
1 INTRODUCTION
The megalencephalic leukoencephalopathy with subcortical cysts (MLC, OMIM 604004) is a rare form of autosomal recessive infantile-onset leukodystrophy (Brignone et al., 2015; Dubey et al., 2018; Hamilton et al., 2018). MLC is a brain channelopathy and has been linked to astrocytes dysfunction (Lanciotti et al., 2013; van der Knaap et al., 2012). Brain edema, subcortical fluid cysts, myelin, and astrocyte vacuolation are the major histopathological hallmarks of MLC (Amin et al., 2021). In majority of MLC cases homozygous mutations in the MLC1 (>75% of patients) or HEPACAM (<20% of patients) genes is the underlying cause of the disorder (Brignone et al., 2015; Capdevila-Nortes et al., 2015; Dubey et al., 2018; Elorza-Vidal et al., 2020a, 2020b; Hamilton et al., 2018; Hwang et al., 2019a; Shi et al., 2019). Moreover, individuals with specific heterozygous mutations (MLC2A variant) in the HEPACAM show mild remitting features (Hamilton et al., 2018). MLC1 encodes a membrane protein, expresses in the brain astrocytes and known to interact functionally with the GlialCAM (a protein product of HEPACAM) in astrocytes at cell–cell junction (Boor et al., 2007; Hwang et al., 2019b; Pérez-Rius et al., 2019; Xie et al., 2012). GlialCAM is a chaperone of MLC1 and it ensures proper localization of MLC1 in the membrane of astrocyte endfeet to establish astrocyte–astrocyte junction (Duarri et al., 2011; Elorza-Vidal et al., 2020a, 2020b; López-Hernández et al., 2011). Moreover, MLC1/GlialCAM complex act as a physiologically relevant functional unit (Pérez-Rius et al., 2019). Mutations in MLC1 results in a series of phenotypic consequences like astrocytes and myelin vacuolation, subcortical cysts, brain edema and macrocephaly (Hwang et al., 2019a, 2019b) (Table S1, Supporting information). Some studies have also reported Schizophrenia and bipolar disorders in patients with MLC1 mutations (Verma et al., 2005). Slow neurological deterioration with cerebellar ataxia and spasticity occurs after several years of onset of disorder (López-Hernández et al., 2011).
The gene MLC1 is located on the long arm of chromosome 22q13.3 and encodes a 377 amino acids seven pass transmembrane protein. MLC1 protein is highly expressed in brain astrocytes, Bergmann glia and ependymal cell lining the ventricles (Leegwater et al., 2001). This plasma membrane protein is a cell membrane transporter and mutations in the MLC1 negatively affect the volume regulated anion channel (VRAC) activity in brain astrocytes and consequently lead to swollen astrocytes (Dubey et al., 2015; Leegwater et al., 2001; Ridder et al., 2011). Therefore, MLC1 mutations cause channelopathy associated brain astrocyte dysfunction. In this study, we recruited a large kindred with multiple affected individuals showing clinical features of ataxia, epilepsy, macrocephaly, and motor function deterioration. Detailed clinical examination and extensive molecular genetic analysis were performed to identify the underlying genetic defects in this family. The objective of this study is to present the clinical features in MLC1 carrier individuals aiming at improved clinical recognition and avoidance of unnecessary genetic testing. This finding will contribute to provide targeted testing for the mutation in MLC1 gene in MLC patients in Saudi population.
2 MATERIALS AND METHODS
2.1 Ethical approval and patients recruitment
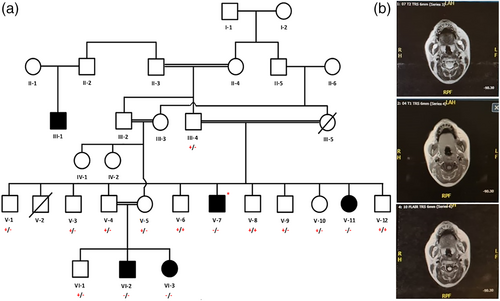
Permission to undertake this study was obtained from the Ethical Review Committee of the College of Medicine, Taibah University Medina, Saudi Arabia. A six generation family with five affected individuals (III:1, V-7, V-11, VI-2, and VI-3) was recruited for this study. Total 15 blood samples were collected in an ethylenediaminetetraacetic acid containing tubes from affected as well as unaffected individuals and their parents. Family elders were informed about the purpose of the study in their local language. Parents of the affected individuals or legal guardian signed the consent for molecular genetic study and publication of genetic data. The pedigree was made using information obtained from the family elders (Figure 1a). Helsinki Declaration of the World Medical Association (October 2013) was followed to handle the human subjects. All affected individuals underwent detailed clinical and radiological examination.
2.2 Genomic DNA extraction for molecular genetic studies
Genomic DNA was extracted from peripheral blood samples of 15 individuals using Qiagen DNA extraction kit (Qiagen, Venlo, The Netherlands). Spectrophotometer (MaestroGen, Hsinchu City 30091, Taiwan) was used to quantify the DNA. Qubit fluorometer was also used to check the DNA concentration and quality.
2.3 Whole genome genotyping
Whole genome SNP genotyping was performed using DNA from 2 affected (VII-2 and VII-3) and 7 apparently unaffected (IV-3, VI-3, VI-4, VI-5, VI-6, VI-8, and VI-12) individuals using Affymetrix GeneChip Human Mapping 250K Nsp array. This array contains probes for 262,000 SNPs. Briefly, 250 ng genomic DNA was digested with a restriction enzyme (Nsp I). The digested samples were ligated with adaptors using T4 DNA ligase followed by dilution of samples with AccuGENE water. Adaptor bound DNA fragments were polymerase chain reaction (PCR) amplified using master mix and thermal cycler. Amplified products were resolved on agarose gel followed by purification and elution. Quantification of the purified product was performed using Qubit followed by fragmentation using DNase I. Fragments were labeled with fluorescent dyes using GeneChip DNA labeling reagent. The labeled samples were loaded onto GeneChip Human Mapping 250K Nsp array. The arrays were placed into a hybridization oven for 16–18 h at 49°C. Array was washed using Fluidics Station 450 followed by staining. Stained arrays were scanned using GeneChip Scanner 3000 7G. Captured images (.dat file) were collected and analyzed. Affymetrix genotyping console software (Affymetrix, Santa Clara, CA, USA) was used to call genotypes.
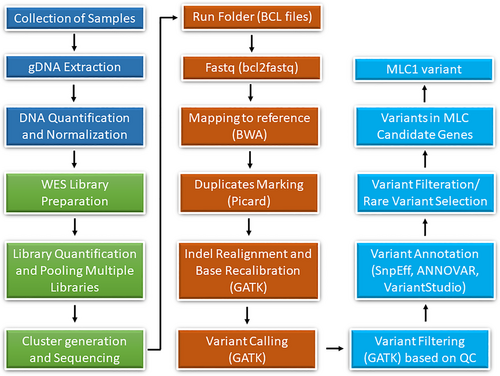
2.4 Whole exome sequencing
DNA of an affected individual (VI-7) was exome sequenced using SureSelect Human All Exon V6 (Agilent). This kit covers most coding RefSeq genes. Briefly, 50 ng of DNA was used for fragmentation followed by ligation of adaptor sequences. Adaptors containing DNA fragments were purified with magnetic beads. Purified fragments were captured and enriched for target regions using whole exome oligonucleotides followed by PCR amplification. Amplified library was quantified with the Qubit fluorometer and library size distribution was measured with the Agilent Bioanalyzer. Paired-end sequencing of libraries was conducted using Illumina V4 high-output flow cell. Cluster generation and DNA sequencing was performed on a HiSeq2500 sequencing machine (Illumina). On average, a read length of 125 bps and the read depth coverage of >150× per target interval was achieved. The raw data were evaluated for read quality with FASTQC software (Babraham Bioinformatics). The Burrows-Wheeler Aligner (BWA), incorporated in BaseSpace with the BWA-MEM algorithm, was used to align FASTQ files to the reference genome. Variants were called using the Genome Analysis Toolkit (GATK, Broad Institute) (Figure 2).
2.5 Variant validation and segregation analysis
Primers flanking variants of interest were designed using Primer3 tool. Sequence of the primers used for Sanger sequencing are Forward-TTCTGGAAGCGCAAATGT and Reverse-GCAGGAGCTTTACTGTCTGG. BigDye terminator (ThermoFisher Scientific) and AB3500 instrument was used to validate the variants of interest. Variants were also Sanger sequenced in all available family members to evaluate the segregation of variants in a family. Sequence chromatograms were aligned with reference sequence using BioEdit tool (version 7.2.6.1).
3 RESULTS
3.1 Clinical evaluation of patients
Four available affected (V-7, V-11, VI-2, and VI-3) and seven carrier (III-4, V-3, V-4, V-5, V-10, V-12, and VI-1) individuals were thoroughly examined by a neurologist in the Madina Maternity and Children Hospital and Saudi German Hospital Medina, Saudi Arabia. All carriers have a history of seizures (at least one episode in life time). Moreover, motor function deterioration was noted in all carriers except VI-1 (Table 1). An affected individual (VI-2) was unremarkable till the age of 8 years, however, he had a single seizure attack at the age of 3 years. At the age of 8, he had repeated seizures and motor functions started deteriorating to extent that he became wheel chair bound at the age of 11. Electroencephalogram analysis was concluded as right temporal epileptogenic dysfunction. Moreover, recent clinical evaluation showed that he is epileptic and has difficulty in communication. Moreover, he developed macrocephaly. Magnetic resonance images (MRIs) of the brain of this individual showed diffused white matter hypo intensity on T1W1 and hyper intensity on T2W1 and FLAIR sequence suggestive of leukoencephalopathy. Posterior fossa and midline structures were normal.
| Pedigree ID | III-4 | V-3 | V-4 | V-5 | V-7 | V-10 | V-11 | V-12 | VI-1 | VI-2 | VI-3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Het | Het | Het | Het | Homo | Het | Homo | WT | Het | Homo | Homo |
| Age (years) | 78 | 24 | 36 | 32 | 47 | 49 | 51 | 53 | 8 | 11 | 14 |
| Gender | M | M | M | F | M | F | F | M | M | M | F |
| Weight (kg) | ND | 48 | 54 | 49 | 44 | 58 | 45 | 84 | 30 | 28 | 30 |
| Age of onset (years) | NA | 28 | 25 | 31 | 4 | 25 | 5 | − | 24 | 3 | 3 |
| Delayed speech development | − | − | − | − | + | − | + | − | − | + | + |
| Seizure | + | + | + | + | + | + | + | − | + | + | + |
| Motor function deterioration | Mild | Mild | Mild | Mild | Severe | Mild | Severe | − | − | Severe | Severe |
| Head circumference | ND | ND | ND | 58 cm (+3.3 SD) | 63 cm (+5.5 SD) | 59 cm (+4.2 SD) | 63 cm (+7.8 SD) | 57 cm (0 SD) | 55 cm (+2.0 SD) | 61 cm (+5.4 SD) | 61 cm (+5.8 SD) |
| Macrocephaly | + | + | + | + | + | + | + | − | − | + | + |
| MRI findings | ND | ND | ND | ND | Leukodystrophy | ND | ND | ND | ND | ND | Leukodystrophy |
- Note: Considered macrocephaly if HC is more than 97 percentile.
- Abbreviations: HC, head circumference; Het, heterozygous; Homo, homozygous; MRI, magnetic resonance image; NA, not available; ND, not done; WT, wild type.
MRI of the brain of affected individual (VI-3) revealed diffused periventricular white matter and disruption of U fibers. White matter abnormal signals and subcortical cysts were suggestive of leukodystrophy. Signal alteration was also observed in T1W1 and TW1/FLAIR sequences. Structural abnormalities as well as acute parenchymal insults were ruled out. Moreover, posterior fossa, ventricles and subarachnoid spaces were normal (Figure 1b).
MRI of an affected individual (V-7) at the age of 47 years revealed mild symmetrical dilatation of the supra tentorial ventricular system. Mild widened cerebral fissures, extra axial CSF spaces, basal cistern and cortical sulci were observed. Diffused white matter hypo intensity on T1W1 and hyper intensity on T2W1 and FLAIR sequence suggestive of leukoencephalopathy were noted. No mass lesions and no intra- or extra axial blood collection were observed. The visualized vessels have normal signal void flow pattern. The physician suggested a brain atrophy and atherosclerotic leukoencephalopathy. The comparative details of the clinical features of our cases with previously published cases of MLC can be found in Table S2.
3.2 Genetic findings
Whole genome SNP genotyping data was analyzed with HomozygosityMapper. Multiple shared regions of loss of heterozygosity (LOHs) were identified in both affected individuals including a 5 Mb region on chromosome 14q, 2 Mb region on chromosome 17p, and a 3 Mb region on chromosome 22q (Figure 3a).
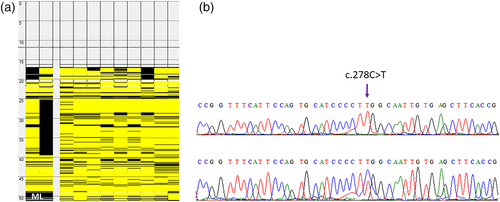
Whole exome data was analyzed by annotating genomic variants with ANNOVAR tool. Several filters were applied to select and prioritize potential disease causing variant. Moreover, a literature search for genes related to leukodystrophy associated with epilepsy/seizure were performed and candidate genes including GBE1, TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, GFAP, LMNB1, ASPA, CYP27A1, EIF2B1-5, SLC17A5, FUCA1, TUBB4A, FAM126A, GALC, L2HGDH, DARS2, EARS2, MLC1, HEPACAM (MLC2), ARSA, PSAP, SUMF1, CSF1R, GJA1, PLP1, GJC2, PEX, POLR3A, POLR3B, POLR1C, RNASET2, HSD17B4, ACOX1, SCP2, ALDH3A2, SOX10, ABCD1 were searched for potential variants. Synonymous and noncoding variants were removed. Variants with minor allele frequency (MAF) of ≤0.01 across 1000 Genomes, ESP6500, ExAC and gnomAD databases were retained. Based on the inheritance pattern of the disease only homozygous variants were considered. Potential sequence variants were evaluated for clinical significance using OMIM, HGMD, and ClinVar databases. Variant conservation was tested with PhyloP. Pathogenicity of the variant was predicted using PolyPhen-2, SIFT, and MutationTaster. A rare missense homozygous variant (NM_015166.3:c.278C>T) in the MLC1 gene was considered as underlying cause of the disease in this family. Interestingly, the gene MLC1 is located in the homozygous stretch on chromosome 22q. This variant is predicted “damaging” by several in silico tools with the CADD score of 29.4 (Table 2). Moreover, it is perfectly segregating with the disease phenotype (Figure 3b). The variant fulfill the PS1, PS4, PP1-S, PM2, PP1-M, PP1, PP3, PP4 evidence categories of ACMG guidelines and, it is therefore, classified as pathogenic.
| S.no | In silico analysis tools | Prediction | Score |
|---|---|---|---|
| 1 | SIFT | Deleterious | 0.00 |
| 2 | MutationTaster | Disease causing | 0.999 |
| 3 | PolyPhen2 | Probably damaging | 0.999 |
| 4 | FATHMM | Damaging | −3.44 |
| 5 | LRT | Deleterious | 0.000 |
| 6 | Condel | Deleterious | 0.886 |
| 7 | CADD | Damaging | 29.4 |
| 8 | M-CAP | Damaging | 0.419 |
| 9 | ClinPred | Damaging | 0.960 |
| 10 | Mutation Assessor | Prediction medium | 2.175 |
| 11 | DANN | Damaging | 0.999 |
| 12 | Meta SVM | Damaging | 0.984 |
| 13 | Meta R | Damaging | 0.891 |
| 14 | PROVEAN | Damaging | −4.18 |
| 15 | VarSome | Pathogenic | PP1, PS1, PS4, PM2, PP3, PP4 |
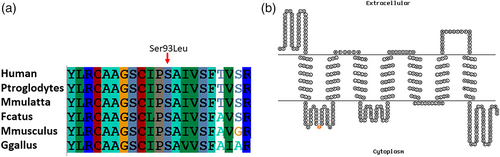
The variant (NM_015166.3;p.(Ser93Leu)) identified in this study is in the first cytoplasmic domain of the seven pass transmembrane protein and the amino acid Serine at position 93 is highly conserved in different orthologues of the MLC1 protein (Figure 4a,b). Heterozygous individuals for the variant (c.278C>T) were further investigated for any clinical manifestation. Clinical history of these individuals revealed that carriers experienced seizures as well as they have mild motor function deterioration.
4 DISCUSSION
Channelopathies are a diverse group of genetic disorders caused by improper functioning of ion channel subunits due to mutation in genes which directly or indirectly encode membrane channels. Ion channels play crucial role in neuronal activity, therefore, channelopathies are responsible for a growing number of nervous system disorders (Brignone et al., 2015). MLC1 encodes a transmembrane protein which regulate other ion channels in the cell membrane of brain astrocytes (Hwang et al., 2019a, 2019b). GlialCAM, encoded by the gene HEPACAM, act in proper folding and transportation of this protein. Moreover, role of GlialCAM in biosynthetic maturation and cell surface expression of MLC1 is well established (Capdevila-Nortes et al., 2015). It is proposed that GlialCAM/MLC1 interaction might modify signal transduction pathways that influence the activity of different proteins, such as VRAC (Elorza-Vidal et al., 2018; Sirisi et al., 2017). Recently, it has been demonstrated that GlialCAM and MLC1 form a heteromeric complex regulating the membrane phase separation of the entire plasma membrane protein signaling cluster, in which MLC1 functions as a regulatory protein. Additionally, this interaction is important to correct plasma membrane location of the signaling cluster (Xu et al., 2021; Pirjo M. Apaja, personal communication). Homozygous mutations in MLC1 and HEPACAM are largely known to cause a specific type of channelopathy called megalencephalic leukoencephalopathy with subcortical cysts (MLC). In rare cases, heterozygous mutations in HEPACAM have been reported to lead to a mild remitting MLC phenotype. However, heterozygous variants in MLC1 gene as an underlying cause of MLC phenotype have not been reported previously.
In this study, we have performed extensive clinical examination and genetic analysis to identify the underlying cause of MLC phenotype in a large kindred. Whole genome SNP genotyping aid in identifying the disease associated shared LOH regions in the genome and whole exome sequencing analysis detected a recurrent disease segregating potentially pathogenic variant (NM_015166.3:c.278C>T) in the MLC1 gene. The variant identified in this study is homozygous in all four affected individuals of the family. The variant affect the conserved amino acid (NM_015166.3;p.(Ser93Leu)) in the cytoplasmic domain of the MLC1 protein (Figure 4b). Few apparently normal individuals of this family were heterozygous for the variant. Detailed clinical examination revealed mild clinical features in carrier individuals including at least a single episode of seizure. Moreover, motor function deterioration was also observed in all carriers except VI-1. This individual is 15 years old and all other carriers have age more than 24 years. Therefore, it is likely that motor function deterioration in heterozygous individuals is age associated. We hypothesize that the heterozygous variant (NM_015166.3:c.278C>T), identified in this study, might disrupt the functional interaction of MLC1 with GlialCAM resulting in mild clinical features. A recent study has analyzed mutant MLC1 protein containing Pro92Ser variant and shown that mutant MLC1 protein has high plasma membrane clearance rate (3–4-fold increased) relative to the wild type MLC1 (Xu et al., 2021). Moreover, accelerated internalization and defective recycling of mutant MLC1 (Xu et al., 2021) relative to the wild type was observed. This account for the fast plasma membrane removal of mutants, mediated by specialized peripheral plasma membrane quality control (PQC) machineries (Wang et al., 2021). Furthermore, it has also been demonstrated that the cytosolic C-terminal tail of GlialCAM promotes the conformational maturation/assembly of the MLC1 in the endoplasmic reticulum (Xu et al., 2021). The variant (Ser93Leu) identified in this study is present very next to the variant (Pro92Ser) discussed above and is located in the cytoplasmic side, therefore, we hypothesize that the mutant (Ser93Leu) MLC1 behave in a similar fashion.
In conclusion, mutations in MLC1 and/or HEPACAM can cause structural and functional composition changes as well as mislocalization of the cluster resulting in variety of neurological phenotypes. This is referred as the signaling cluster misalignment and changes in the tethering (Pirjo M. Apaja, personal communication). This molecular mechanism could explain clinical phenotype resulting due to the homozygous/heterozygous mutations in the MLC1. However, one can assume that the differences in severity of phenotypes depends on the extent of malfunctioning of the signaling cluster.
ACKNOWLEDGMENTS
We thank patients and the other members of the family for their cooperation throughout the study. We are also very thankful to Pirjo M. Apaja (University of Adelaide, Australia) for her comments on the interaction of GlialCAM and MLC1 and signaling cluster misalignment due to mutation in MLC1 gene.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
AUTHOR CONTRIBUTIONS
A.A., G.A., and S.B.; investigated the project. S.A.B., and G.A.; wrote the manuscript. SB conceptualized the study, reviewed and edited the manuscript. M.A., and F.A.; recruited subjects for the study and performed clinical examination.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the College of Medicine, Taibah University Medina, Saudi Arabia.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study. Moreover, written informed consents have been obtained from all the patients to publish this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.