Clinical and molecular response to dasatinib in an adult patient with Penttinen syndrome
Abstract
Penttinen type of premature aging syndrome is an autosomal-dominant disorder that can be caused by the c.1994T>A pVal665Ala pathogenic variant in platelet-derived growth factor receptor-B (PDGFRB). Imatinib, a receptor tyrosine kinase (RTK) inhibitor, has been used in Penttinen syndrome (PS) patients with good results. A 21-year-old male presented shortly after birth with a prematurely aged appearance with distinctive facial features and cutaneous atrophy with hypertrophic scar-like lesions. Generalized brachydactyly with acro-osteolysis was observed. Flexion contractures limited his daily activities. Cognitive impairment was not present. Genetic testing found a heterozygous variant c.1994T>A pVal665Ala in exon 14 of PDGFRB. A diagnosis of PS was made and imatinib treatment was started with partial response. After lack of further improvement, in vitro molecular studies with imatinib and dasatinib showed that the Val665Ala variant had greater sensitivity to dasatinib than imatinib. This was seen examining levels of P-PDGFRB directly and on downstream ligands P-AKT and P-STAT. Improved clinical response was observed after treatment with dasatinib. We report a new case of PS with clinical and molecular response to dasatinib after incomplete response to imatinib. Our work provides further molecular and clinical evidence of RTK inhibitors' efficacy in this rare disorder.
1 INTRODUCTION
Progeroid syndromes are a group of genetic disorders characterized by an appearance of premature aging without associating premature death. Penttinen type of premature aging syndrome (Penttinen syndrome; PS) (OMIM#601812) was originally described by Penttinen et al. (1997). This rare progeroid syndrome is characterized by a prematurely aged appearance with distinctive facial features, acro-osteolysis and atrophic skin with hypertrophic scar-like lesions (Johnston et al., 2015; Penttinen et al., 1997; Zhang et al., 2018; Zufferey et al., 2013). It is an autosomal-dominant disorder that can be caused by the c.1994T>A Val665Ala pathogenic variant in platelet-derived growth factor receptor-B (PDGFRB). Johnston et al. (2015) showed that the missense variant, located in the tyrosine kinase region of PDGFRB, led to ligand independent phosphorylation of PDGFRB and activation of downstream signaling molecules STAT3 and PLCy in transfected HeLa cells.
PDGFRB is known to bind PDGF-B and PDGF-D dimers, promoting growth of mesenchymal cells. Pathogenic variants in PDGFRB, either constitutional or mosaic variants, have been linked to several disorders. Loss-of-function pathogenic variants cause idiopathic basal ganglia calcification type 4 syndrome (OMIM#615007) (Johnston et al., 2015) while heterozygous germ-line activating pathogenic variants are involved in several disorders: infantile myofibromatosis (OMIM#228550; Arg561Cys, Pro660Thr, Asn666Lys, Pro560Leu, and Lys567Glu) (Arts et al., 2017; Lepelletier et al., 2017; Murray et al., 2017), PS (OMIM#601812; Val665Ala, Asn666Ser), Kosaki overgrowth syndrome (KOS) (OMIM#616592; Pro584Arg, Trp566Arg) (Gawliński et al., 2018; Minatogawa et al., 2017), Ocular-pterygium-digital keloid dysplasia (OPDKD) (Asn666Tyr) (Bredrup et al., 2021) and in a patient with a combination of PS and KOS features (Asn666His) (Pond et al., 2018). In addition, postzygotic (mosaic) activating pathogenic variants have also been described: a man with segmental overgrowth, atrophic skin and multiple intra and extracranial fusiform aneurysms (Karasozen et al., 2019) and a patient with infantile myofibromatosis and some PS and KOS-related features (Guimier et al., 2019).
Wenger et al. (2020) recently published case series of 12 patients with activating variants in PDGFRB. The authors suggest a single disease spectrum that they call PDGFRB activating variant spectrum (PAVS) disorder, with two subgroups according to disease severity and multisystem affection. PAVS1 includes a more benign form represented by the previously called “infantile myofibromatosis,” with multiple myofibromas and aneurysm formation. Five different activating pathogenic variants in the PDGFRB have been described so far in this group. PAVS2 comprises a group of complex multisystem progressive disorders affecting mainly cerebrovascular and connective tissues, including PS, KOS, and related progeria-like variants, with eight different activating pathogenic variants so far (Wenger et al., 2020). PAVS2 common features include overgrowth, progressive vascular dysplasia, progressive leukoencephalophaty and unusual abnormalities of brain, bone, skin, and connective tissues. In vitro functional studies performed suggest that the germ-line variants found in PAVS1 patients are less activating than those associated with PAVS2, thus maybe explaining the more severe phenotypes found in the latter group. Recently, temperature-dependent autoactivation was found to be associated with clinical variability in PDGFRB variants affecting codon Asn666 (Bredrup et al., 2021), suggesting that also other factors are important in determining clinical outcome of activating PDGFRB variants.
Gain of function pathogenic variants promote the active conformation of PDGFR in the absence of its ligand, resulting in constitutive activation of downstream signaling pathways (Arts et al., 2017). Several activating pathogenic variants in PDGRFB are sensitive to receptor tyrosine kinase (RTK) inhibitors in vitro (Abarca et al., 2014; Arts et al., 2017; Bredrup et al., 2021, 2019; Pond et al., 2018; Wenger et al., 2020) and in vivo (Martignetti et al., 2013; Pond et al., 2018; Wenger et al., 2020), with variable results. In patients with infantile myofibromatosis, clear and rapid reduction in size of myofibromas has been observed (Mudry et al., 2017; Wenger et al., 2020). One PS patient (Val665Ala) (Wenger et al., 2020) and another PS-related patient (Asn666His) (Pond et al., 2018) have also been treated with imatinib. The response is more difficult to evaluate in these patients due to the multisystem affection, but connective tissue-related symptoms (mainly flexion contractures and cutaneous changes) improved. A patient with OPDKD (Asn666Tyr) found sensitive to imatinib in vivo had a clinical deterioration on imatinib treatment (Abarca et al., 2014). Herein, we describe a new case of PS (Val665Ala) with partial molecular and clinical response to imatinib, with further improvement after dasatinib treatment.
2 METHODS
The patient and his family were invited to participate in the study and were included after informed consent, including specific consent to publish facial photographs.
2.1 Clinical report
The patient was a 21-year-old male delivered at term after an uneventful pregnancy from healthy, nonrelated parents. He had a healthy older brother. At birth he was phenotypically normal, but 2 months later he developed progressive hydrocephalus, requiring a ventriculoperitoneal shunt. Since then, he slowly developed features of premature aging that gave him an aged appearance. His psychomotor development remained completely normal. He was very tall (197 cm) and thin (67 kg), with generalized lipoatrophy. He had characteristic facies (Figure 1) with frontal bossing, proptosis with loss of periorbital fat, malar hypoplasia with retrognathia and microstomy, all together causing malocclusion which required surgical correction. He also had convex nasal ridge. Eruption of permanent teeth was delayed. He had sparse, blond hair, and atrophic skin, with marked venous pattern. There were reticulated and thickened scar-like lesions on the dorsum of his hands, feet, elbows, and knees. Generalized brachydactyly with short and broad distal phalanges was observed on hand and feet. Flexion contractures of the fingers limited his daily activities.
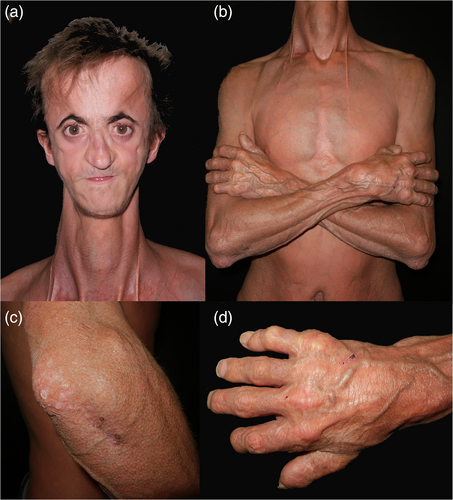
Cranial computed tomography and magnetic resonance imaging demonstrated open anterior and posterior fontanels and sagittal suture; a large arachnoidal cyst in the posterior fossa with cerebellar hypoplasia, another arachnoidal cyst in the left temporal area and leukoencehalopathy of the bilateral periventricular and supratentorial white matter. No basal ganglia calcifications were found. His skeletal survey showed lumbosacral hyperlordosis, moderate osteopenia and thin and bowed tibias with acro-osteolysis of hands and feet. Electrocardiogram and echocardiogram including Doppler studies of supra-aortic trunks were completely normal. Ophthalmologic examination showed bilateral inferonasal corneal pannus with corneal opacities and superficial punctate keratitis. Clinical testing showed moderate mixed hearing loss. There was no hypogonadism and external genitalia were normal. Blood tests ruled out metabolic disturbances. Intellectual development was normal, and he is currently taking a university degree. A skin biopsy of one of the nodules showed epidermal atrophy, hyperkeratosis, and dermal fibrosis.
Genetic testing for Hutchinson-Gilford progeria, Werner syndrome, and other progeroid disorders (acromandibular dysplasia, Nestor-Guillermo syndrome) was performed. Sequencing of LMNA (OMIM#150330), ZMPSTE24 (OMIM#606480), RECQL2 (OMIM#604611), and BANF1 (OMIM#614008) were normal. Genetic testing with next generation sequencing identified a heterozygous variant (c.1994T>A Val665Ala) in exon 14 of PDGFRB. Sanger sequencing ruled out other family members involvement, being a de novo variant (Figure 2). A diagnosis of PS was made and treatment with imatinib was offered. All potential side effects were extensively discussed. Baseline blood cell counts, blood chemistry, and coagulation tests were performed before initiation. At age 21 years and 3 months imatinib was started at a dose of 200 mg daily. Monitoring of adverse effects with clinical interview, blood cell count and blood chemistry was made after 3 weeks. No side effects nor cytopenias were observed, so imatinib was escalated to 400 mg daily (standard dose for treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia). Two months after starting treatment, he reported improvement of contractures of both hands, being able to perform hand exercises that had not been able to do before. No cytopenias or infectious complications were observed after 16 months of treatment. He had transient fatigue that resolved without dose modifications. On follow-up visits, not further improvement was seen. In the setting of the COVID-19 pandemic crisis, imatinib therapy was stopped in June 2020. After this, further progression of flexion contractures was observed and imatinib was restarted December 2020. Very slight changes were observed after 1 month. After careful consideration and given the results of the molecular analysis showing higher sensitivity to dasatinib (further discussed below), treatment switch to dasatinib 70 mg daily, was performed in February 2021, with dose escalation to 140 mg daily in June 2021. Clinical improvement—manifested as decrease in flexion contractures and cutaneous induration and increase in range of movements of hands and feet—was noted 1 month after starting treatment. Patient-reported outcome using PROMIS® questionnaires for upper extremities reported improvement after dasatinib. Follow-up time is currently 8 months and the only observed side effect has been mild transient asthenia.
2.2 In vitro pharmacogenetic studies
Fibroblasts from the affected individual and healthy controls were cultured in Dulbecco's modified Eagle's medium with high glucose (Lonza) supplemented with 10% fetal calf serum, penicillin, streptomycin, and glutamine. The cells were transferred to serumfree medium and either left untreated or treated with 0.1 or 1.0 μM imatinib or with dasatinib (#STI571 and #S1021, both Selleckchem) for 6 h before they were harvested. Analysis of total phosphorylated PDGFRB were performed by ELISA analysis (DuoSet® IC PDGFRβ kit, #DYC1767-2; R&D systems). In this kit, an immobilized phospho-PDGFRβ antibody binds PDGFRβ (both phosphorylated and unphosphorylated) in the cell lysate. After washing, a horseradish peroxidase-conjugated antibody against phosphorylated tyrosine is used to detect total receptor phosphorylation. Levels of downstream effector proteins (phospho-Ser473-AKT (#4060), AKT (#4691), phospho-Tyr70-STAT1 (#7649), and STAT1 (#9172) (all Cell Signaling Technology) were determined by immunoblot analysis as previously described (Bredrup et al., 2019).
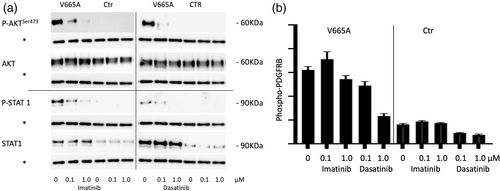
Western blot analysis showed clear P-AKT and P-STAT1 upregulation in patient fibroblasts. After 6 h of treatment with 0.1 μM imatinib, levels of P-AKT and P-STAT1 were downregulated, although not completely normalized. Further reduction was seen at increased levels of imatinib (1.0 μM) (Figure 2a). Following limited clinical therapeutical response, levels of phosphorylated PDGFRB were determined in patient's fibroblasts using ELISA analysis (Figure 2b). Basal increased levels were observed, with scarce modifications after imatinib treatment. Dasatinib treatment showed substantially higher reductions in levels of phospho-PDGFRB and of downstream signaling partners (Figure 2a,b). All findings were repeated in three independent experiments.
HEK293 cells were transduced with wild-type (WT) or the Val665Ala variant similarly to as previously described (Bredrup et al., 2019). Cells transduced with the WT vector were stimulated for 20 min with 12.5 ng/ml PDGFRB-BB (Sigma-Aldrich) according to the manufacturer's instructions. They were then either left untreated or treated with 0.1 or 1.0 μM imatinib or dasatininb. While both drugs were effective in reducing levels of phospho-PDGFRB dasatinib was found to be much more effective in reducing ligand-dependent PDGFRB activation. Cells transduced with the Val665Ala variant were more sensitive to treatment with dasatininb, similar to the drug response in human fibroblasts (Figure S1).
3 DISCUSSION
Although described as distinct clinical entities, there is an undeniable clinical overlap between PS and KOS. Even though PS is classified in the progeroid group of conditions, several PS patients, including our own, showed an accelerated postnatal growth with height above +3SD (Zufferey et al., 2013). Cutaneous and articular alterations are also described in both entities, although being much more severe in PS. Abnormal skull ossification is also present in both, but more prominent in KOS. Finally, arachnoid cysts have also been reported in both PS (Case 2 in Zufferey et al. (2013) and our patient) and KOS (Case 1 in Minatogawa et al. (2017) and Gawlinski et al. (2018)). Interestingly, our patient also developed postnatal hydrocephaly requiring a ventriculoperitoneal shunt, as Case 2 in Minatogawa et al. (2017). In addition, myofibromas have been linked to KOS patients and to the PS-related patient described by Pond et al. (2018). This same patient also had acro-osteolysis changes with progressive brachydactyly and contractures and atrophic skin with palmoplantar thickening and scar-like lesions, features common to PS (Pond et al., 2018).
Special interest has been focused in the use of RTK inhibitors for the treatment of these disorders, and some of these pathogenic variants have proven to be sensitive to imatinib both in vitro (Arts et al., 2016; Bredrup et al., 2019; Pond et al., 2018) and in vivo. Significant clinical responses have been observed when treating multicentric myofibromas with sunitinib and imatinib (Mudry et al., 2017; Wenger et al., 2020), with regrowth of myofibromas after treatment interruption (Wenger et al., 2020). A child with PS and KOS overlapping features with the Asn666His variant was treated by Pond et al., with rapid improvement of contractures and decrease of coarse facial features (Pond et al., 2018). In contrast, a patient with OPDKD did not respond to imatinib treatment, despite the PDGFRB variant (Asn666Tyr) being sensitive to treatment in vivo (Bredrup et al., 2021). More recently, a patient with PS (pVal665Ala, Patient 3 on Johnston et al.) was also started on imatinib, with decreased hand pain, improved skin turgor, reduction of scars, and decreased conjunctival injection, only 2 months after initiating treatment (Wenger et al., 2020). Our patient also experimented a decrease in flexion contractures in the first weeks after starting imatinib, with further improvement seen with dasatinib.
Even though Johnston et al.'s patient carries the same Val665Ala pathogenic variant as ours, differences in therapeutic response are evident. Possible reasons could be that the advanced age of our patient might have contributed to the limited response, as degenerative features caused by the disease cannot be reversed. Therefore, aim of treatment in our case was to prevent further progression of damage. Also, even though western blot analysis in our patients' fibroblasts showed clear P-AKT and P-STAT1 downregulation after imatinib treatment, phospho-PDGFRB levels showed little modifications. Imatinib targets other tyrosin kinases besides PDGFRB, so P-AKT and P-STAT downregulation could have been caused by inhibition of other signaling pathways. Our molecular studies suggest that the Val665Ala pathogenic variant is significantly more sensitive to dasatinib, both indirectly on downstream ligands (P-AKT and P-STAT1) and directly (P-PDGFRB). The data needs to be interpreted with some caution as dasatinib was found to be more effective also in treating ligand-dependent activation. However, imatinib is reported to have an IC50 value for inhibiting PDGF at 0.1 μM which corresponds to what we found when treating ligand-dependent PDGFRB activation (Figure S1). The lack of a significant response in fibroblasts and transduced cells carrying the Val665Ala variant to treatment with 0.1 μM imatinib therefore strongly suggests that this variant has little sensitivity to imatinib. Higher sensitivity to dasatinib was supported by a further clinical improvement after dasatinib treatment.
4 CONCLUSION
We provide further proof that RTK inhibitors could be useful in treating PS. Our results suggest that targeted therapy with RTK inhibitors improves quality of life of these patients, and that dasatinib treatment is a therapeutic option either as first line or after imatinib resistance. Future studies with long-term follow-up data are needed to determine the effect of early and long-term treatment on disease development.
ACKNOWLEDGMENT
The authors thank the patient and his family for their collaboration in this study.
CONFLICT OF INTERESTS
The authors do not declare any conflict of interests.
AUTHOR CONTRIBUTIONS
Helena Iznardo: Conceptualization (equal), writing—original draft (lead), writing—review and editing (equal), data curation (equal), investigation (equal), validation (equal), visualization (equal). Cecilie Bredrup: Data curation (equal), reviewing and editing (equal), investigation (lead), validation (equal), visualization (equal). Sara Bernal: Data curation (equal), reviewing and editing (equal), investigation (equal), validation (equal), visualization (equal). Titas Gladkauskas: Data curation (equal), reviewing and editing (equal), investigation (equal), validation (equal), visualization (equal). José-Manuel Mascaró Jr: Data curation (equal), reviewing and editing (equal), investigation (equal), validation (equal), visualization (equal). Esther Roé: Data curation (equal), reviewing and editing (equal), investigation (equal), validation (equal), visualization (equal). Eulalia Baselga: Conceptualization (lead), supervision (lead), project administration, metohodology (lead), data curation (equal), reviewing and editing (equal), investigation (equal); validation (equal), visualization (equal).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, HI, upon reasonable request.