A new case of osteogenesis imperfecta type VIII and retinal detachment
Funding information: Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Number: 306861/2019-4; FAPERGS/MS/CNPq/SESRS, Grant/Award Number: 17/2551-0001437-5
Abstract
Osteogenesis imperfecta (OI) type VIII (OMIM: 610915) is a rare autosomal recessive disorder characterized by white sclerae, severe growth deficiency, and bone fragility. This condition results from pathogenic variants of P3H1, a gene that codes for P3H1, an important protein involved in the prolyl-3-hydroxylation complex required for collagen type I folding. Here, we described a woman with OI type VIII due to a homozygous mutation of c.1914+1G>C (NM_001243246.1) in P3H1 and retinal detachment. We compared our case to five severe OI and retinal detachment cases reported in the literature. The only case previously reported with a molecular diagnosis had a similar mutation in P3H1 c.1914+1G>A and a giant retinal detachment. We suggest that individuals with OI type VIII should be submitted to careful fundoscopic examination.
1 INTRODUCTION
Osteogenesis imperfecta is a heterogeneous disorder characterized by low bone mass, leading to fractures, and bone deformities. Additional clinical findings are short stature, blue sclerae, dentinogenesis imperfecta, and cardiorespiratory abnormalities. Approximately 85% of cases occur due to mutations in COL1A1 or COL1A2 genes leading to collagen type I quantity or structural defects. Type I collagen is a heterotrimer containing two procollagen α1 and one α2 polypeptide chains that undergo post-translational modifications in the endoplasmatic reticulum (Marini et al., 2014). This initial modification requires a prolyl-3-hydroxylation complex formed by Cartilage-associated protein (CRTAP), prolyl 3-hydroxylase 1 (P3H1), and cyclophilin B (CyPB). The complex is responsible for modifying the Proline 986 residue in unfolded procollagen α1 and Proline 707 in α2 to 3-hydroxyproline (Weis, et al., 2010).
These complex proteins and others involved in mineralization, collagen folding, crosslinking and chaperoning and osteoblast development lead to autosomal recessive OI (Marini et al., 2014; Tonelli et al., 2020).
P3H1 was first described as the matrix proteoglycan leprecan and is encoded by the P3H1 (prolyl 3-hydroxylase 1) gene. Null P3H1 alleles cause type VIII OI, and several cases had been reported in the literature (Cabral et al., 2007; Marini et al., 2010; Pepin et al., 2013). The most common P3H1 allele causing OI is c.1080+1G>T, a founder mutation originating in West Africa that has been reported in patients of African-American ancestry (Cabral et al., 2012). Two Brazilian cases had been reported with this mutation by two independent groups (Barbirato et al., 2015; Trancozo et al., 2019).
Scollo et al. (2018) reported on a type VIII OI with P3H1 homozygous c.1914+1G>A and bilateral giant retina tears. We report on the second case with a similar mutation in P3H1.
2 CASE REPORT
This 28-year-old female was included in the Brazilian Osteogenesis Imperfecta Network Study, an IRB approved study (#47277215.8.1001.5327) for clinical and molecular study of Osteogenesis Imperfecta, and an informed consent form was signed. She is the only daugther of Italian ancestry of a distant consanguineous family, where both grandparents lived in a small countryside town. She was born by C-section due to the diagnosis of bone dysplasia on prenatal ultrasound. Her birth weight was 2,600 g, and the birth length was 42 cm. At birth, multiple limb deformities and consolidated fractures were reported, and the diagnosis of OI was made. She had multiple fractures, especially in the lower and upper limbs, until 5 years old. At 9 years old, she was submitted to surgery for scoliosis and at 24 years old to femur surgeries due to fractures. She had a severe motor delay, holding her head at 12 months old and sitting unsupported at 30 months old. At 28 years old, she was able to walk very short distances with a walker. She was treated with intravenous pamidronate from 10 to 14 years old, and afterward, it was replaced by oral bisphosphonate. At physical examination, she had severe short stature (length = 87 cm; z-score = −11.5) white sclerae, no dentinogenesis imperfecta, severe deformity of the upper and lower limb, and thoracolumbar scoliosis. DEXA showed lumbar bone mineral density (BMD) = 1.241 (z-score = 1.8), femur = 0.661 (z-score = −1.8), and total body = 0.891 (z-score = −0.1). X-rays showed severe scoliosis, asymmetry of lower limbs, thin bones, and severe deformities on femurs, tibias, and fibulas (Figure 1a–d).
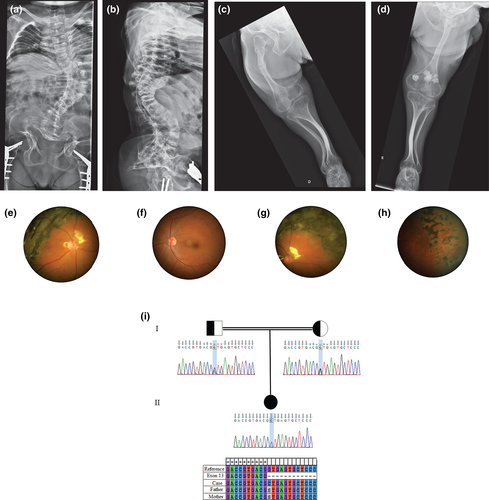
At the age of 14, she presented with decreased vision in her right eye for a few months. The patient exhibited low myopia in the right eye (RE) and moderate myopia in the left eye (LE), as well as high with-the-rule astigmatism in both eyes (−1.50/−3.25 × 15°; −4.00/−3.00 × 180°). The best spectacle-corrected vision at first visit was RE 0.70 logMAR (20/100) and LE 0.10 logMAR (20/25). Upon initial examination, her anterior segment biomicroscopy presented no alteration. In the RE, the posterior segment examination revealed rhegmatogenous retinal detachment with underlying subretinal fibrosis in the temporal intermediate periphery associated with macula-off detachment. In the LE, fundoscopy revealed vitreous traction in the upper and temporal retinal periphery. Following this examination, a 360° circumferential pattern argon laser photocoagulation was performed in the LE retinal periphery. Surgery for RE consisted of 25-gauge pars plana vitrectomy (PPV) tamponaded with silicone oil. Retinal stability was achieved in both eyes for more than 8 years follow-up, but a persistent temporal proliferative vitreoretinopathy (PVR) and residual submacular fluid were still observed in the RE (Figure 1e–h). Progressive peripheral retinal thinning adjacent to photocoagulation was observed during this period in the LE. The last visit showed visual acuity of RE 1.00 logMar (20/200) and LE 0.00 (20/20).
Genetic analysis was performed by the Ion Torrent PGM platform. The panel covers 99.6% of the coding region of 18 genes: COL1A1, COL1A2, CRTAP, P3H1, PPIB, WNT1, TMEM38B, SERPINH1, BMP1, SP7, SERPINF1, FKBP10, SMPD3, CREB3L1, PLOD2, P4HB, PLS3, IFITM5, and the 5′UTR region of IFITM5. Data were processed using Torrent Suite and Ion Reporter (version 5.0; Life Technologies). A homozygous variant c.1914+1G>C (NM_001243246.1) was identified on P3H1, confirming type VIII OI. A heterozygous mutation was identified in both parents by Sanger sequencing (Figure 1-i).
3 DISCUSSION
Abnormalities of the cornea and sclerae have been widely recognized in OI. A homogenous thin sclerae had been reported in OI, being thinner in type I OI when compared to other types (Magalhaes et al., 2018).
Abnormalities of the retina had been reported in a few cases, as presented in Table 1 (Madigan et al., 1994; Church & Winder, 2006; Jonisch & Deramo, 2011; Fleissig & Barak, 2017; Scollo et al., 2018). Of those five cases, one was a type VIII OI and the remaining had no molecular analysis performed. However, one case was described as severe OI and another as type III OI. All cases with no molecular analysis had pale blue or white sclerae, and retinal detachment was reported in adolescence or adulthood.
| Madigan et al., 1994 | Church & Winder, 2006 | Jonisch & Deramo, 2011 | Fleissig & Barak, 2017 | Scollo et al., 2018 | Present report | |
|---|---|---|---|---|---|---|
| Gender | Female | Female | Male | Female | Male | Female |
| Age at retinal detachment | 13 | 31 | 64 | 37 | 9 | 14 |
| OI type | NR | Severe | NR | III | VIII | VIII |
| Mutation | NR | NR | NR | NR | P3H1 Homozygous c.1914+1G>A |
P3H1 Homozygous c.1914+1G>C |
| Sclera | Blue | Pale blue | White | Bluish | NR | White |
| Short stature | NR | + | NR | Severe | Small | Severe |
| Fractures | Multiple | Multiple | 55 | Multiple | Multiple | Multiple |
| Deformities | NR | Skull | NR | Severe | Skull | Severe |
| Right eye | Prominent cystoid degeneration of the retina in the temporal quadrant | – | Supero temporal periphery retinal tear | Inferior tempora retinal detachment | Macular off retinal detachment with giant retinal tear | Rhegmatogenous retinal detachment with underlying subretinal fibrosis in the temporal intermediate periphery associated with macula-off detachment |
| Left eye | Prominent cystoid degeneration of the retina in the temporal quadrant | Macular on retinal detachment with an inferior hole | Superonasal retinal tear | Inferior temporal branch vein occlusion | Giant retinal detachment | Vitreous traction in the upper and temporal retinal periphery |
A giant retinal detachment was observed in this 9-year-old boy with type VIII OI presenting a homozygous mutation c.1914+1G>A in the P3H1 gene (Scollo et al., 2018). This is a similar mutation reported in the present case (c. 1914+1 G>C). The c.1914+1G>A mutation had been previously reported in a 10-year-old boy as compound heterozygous with c.1244dup (p. Arg416Thrfs*40) in a large series of P3H1 mutations defects; however, no clinical finding is described. This mutation predicts the disruption of the IVS 13 donor site (Pepin et al., 2013).
Retinal detachment surgery, such as scleral buckle, might not be appropriate for all patients with OI because the sclerae are thin and susceptible to perforations (Fleissig & Barak, 2017). Suturing the sclerae in standard vitrectomy also carries a greater than usual risk of perforation. Smaller wound incision without the need for scleral sutures in 25-gauge vitrectomy might be preferred in cases of significant scleral thinning, such as OI (Jonisch & Deramo, 2011). Pneumatic retinopexy and external drainage of subretinal fluid followed by cryotherapy on a postoperative day one have also been described as the successful treatment of a retinal detachment in OI (Madigan et al., 1994). Unfortunately, genetic analysis was not available in these cases. We believe that molecular study might guide ophthalmic fundoscopic monitoring and prophylactic treatment in OI patients.
As verified by this report, we suggest that every type VIII OI (P3H1 gene variation) patient must be submitted to careful fundoscopic examination. If any alteration in the retinal periphery is observed, prophylactic 360° peripheral pan-retinal photocoagulation (PRP) is indicated.




