Chimerism involving a RB1 pathogenic variant in monochorionic dizygotic twins with twin–twin transfusion syndrome
Abstract
We report the first case of blood chimerism involving a pathogenic RB1 variant in naturally conceived monochorionic-dizygotic twins (MC/DZ) with the twin–twin-transfusion syndrome (TTTS), presumably caused by the exchange of stem-cells. Twin A developed bilateral retinoblastoma at 7 months of age. Initial genetic testing identified a de novo RB1 pathogenic variant, with a 20% allelic ratio in both twins' blood. Subsequent genotyping of blood and skin confirmed dizygosity, with the affected twin harboring the RB1 pathogenic variant in skin and blood, and the unaffected twin carrying the variant only in blood.
1 INTRODUCTION
Hereditary retinoblastoma is an autosomal dominant cancer predisposition syndrome caused by pathogenic variants in RB1. Bilateral retinoblastoma is almost always associated with a germline pathogenic variant in RB1.
Genetic chimerism is a phenomenon in which one or more genetically distinct cell lines from different zygotes, coexist in a single organism. The theory of hematopoietic chimerism through intra-placental blood exchange was raised more than 50 years ago by studying concordant leukemia in MZ twins. Clarkson and Boyse in 1971 proposed that higher concordance of leukemia in MZ twins is caused by the exchange and permanent colonization of hematopoietic stem-cells between twins. They speculated that both homozygosity and placental status might play roles in concordant leukemia in MZ twins (Clarkson & Boyse, 1971).
More recent studies have also proposed that hematopoietic stem cell exchange between monochorionic twins is possibly the cause of chimerism in MC/DZ twins (Dziegiel et al., 2017; Fumoto et al., 2014). The first case of chimerism in cells other than blood cells in MC/DZ twins was reported in 2014. The authors hypothesized that the presence of chimeric cells in buccal epithelial cells is from the migration of the ectopic differentiated hematopoietic cells to the buccal membranes (Fumoto et al., 2014).
Monochorionicity was previously considered to be a distinctive feature of monozygosity. The first human blood-group chimera in DZ twins reported in 1953 (Dunsford et al., 1953). A large study by Van Dijk and colleagues showed 8% blood-chimerism in DZ twin pairs, suggesting that blood chimerism can also happen in DZ twins and is indeed not a rare event (Van Dijk, Boomsma, & de Man, 1996). More recently, Peters and colleagues published a review of 31 reported cases of monochorionic, dizygotic (MC/DZ) twins, 82.1% of which were conceived with the use of assisted reproductive technologies, and 17.9% (five cases) were products of natural conception (Peters et al., 2017). Among these reported cases of MC/DZ twins, 90.3% had some degree of detectable chimerism (Peters et al., 2017).
Twin–twin transfusion syndrome (TTTS) is a condition that develops almost exclusively in monochorionic twins when there is a pathologic circulatory imbalance in placental vascular anastomoses (Umur, van Gemert, Nikkels, & Ross, 2002). Complications of TTTS include oligohydramnios in the donor twin, and polyhydramnios and cardiac volume overload in the recipient twin due to unequal blood sharing. Chimerism can add to challenges faced in these unusual twinning.
We report a case of blood chimerism involving a pathogenic variant in RB1, in both MC/DZ twins, caused by a shared placenta. To our knowledge, this is the first report of naturally conceived MC/DZ twins with blood chimerism for a pathogenic variant in RB1. Herein, we discuss the diagnostic process of this unusual case and its implications for clinical management.
2 CLINICAL REPORT
The probands were born at 35 weeks of gestation via cesarean section to a G1P2 mother after natural conception. The pregnancy was complicated by TTTS, diagnosed prenatally by ultrasound in the second trimester. The monochorionicity was diagnosed at 8 weeks of gestation by ultrasound and later was confirmed by histology. This twin pregnancy was initially assumed to be monozygotic due to monochorioncity. Twin A was diagnosed with bilateral retinoblastoma at 7 months of age, prompting a genetic investigation. Initial sequencing performed on the blood of affected twin, using NGS sequencing, identified a heterozygous RB1 c.1445_1446del (p.Phe482fs) pathogenic variant with about 20% allelic ratio. The diagnostic lab report described “an unusual pattern in the data suggestive of blood chimerism.” The unaffected twin's blood (twin B) found to have the same allelic ratio for the RB1 variant.
To confirm and clarify the results after obtaining informed consent from probands' parents, we collected peripheral blood and skin biopsy samples of both twins. A SNP analysis was performed on both twins' blood and cultured fibroblasts to clarify the twins' zygosity status. We used common (more than 2% minor allele frequency), biallelic SNPs from the 1000 Genomes Project (Genomes Project et al., 2015), intersecting with our hereditary cancer sequencing panel. After filtering for SNP genotypes with depth >30 and genotype quality >50 in all four samples (blood and skin sample of both twins), we had 432 SNPs. We then filtered for SNPs with total allelic fraction in the four samples >0.01 and <3.99 to keep only those SNPs that are likely to be different between the samples and thus informative. This eliminated variants that were homozygous reference or homozygous for the alternate allele, in all four samples. We were left with 217 informative SNPs and one pathogenic variant (Table S1). We binned allelic fractions by 0.1. Variants were sorted by allelic fraction bins and plotted as a heatmap (Figure 1).
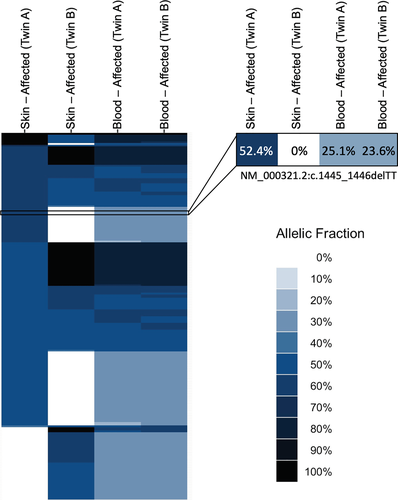
The result confirmed that the twins are, in fact, dizygotic, with twin A harboring the RB1 pathogenic variant in the skin and blood, and twin B displaying the variant only in the blood. Repeated analysis of the blood for both twins showed the same allelic ratio for the RB1 variant.
The family history was noncontributory, and the RB1 pathogenic variant identified in probands was not found in either parent. Although this could suggest that the variant occurred de novo, germline mosaicism in parents could not be excluded.
The affected twin underwent intra-arterial chemotherapy to the right eye and indirect ophthalmoscopic laser therapy to both eyes. Both twins have been evaluated by an ophthalmologist and are receiving standard care for management and surveillance of retinoblastoma. The unaffected twin, now 3 years old, remains clinically asymptomatic.
3 DISCUSSION
Here, we report the first case of naturally conceived MC/DZ twin chimeras for a pathogenic variant in RB1. We posit that this chimerism is possibly caused by the exchange of the stem-cells between twins through their shared placenta. Twin A with bilateral retinoblastoma had the RB1 variant in her blood and skin. Twin B had the variant only in her blood and remained cancer-free. The absence of the pathogenic RB1 variant in the unaffected twin's skin suggests Blood-Confined chimerism, but we cannot exclude cryptic chimerism in her other organs.
Penetrance of retinoblastoma for RB1 null variants is >99% by age 5 years. Germline pathogenic variants in RB1 can increase the risk of other cancers, such as pinealoblastomas, osteosarcomas, rhabdomyosarcomas, and melanomas (Lohmann & Gallie, 1993). Children with a germline pathogenic variant in RB1 are required to have serial fundoscopic examinations as frequently as monthly in their first year of life for early detection and management of retinoblastoma (Skalet et al., 2018). Besides, screening for pineoblastoma and second primary tumors should be considered (Kamihara et al., 2017). Furthermore, all affected individuals should receive counseling for family planning when they reach childbearing age (Kamihara et al., 2017; Skalet et al., 2018). Although guidelines recommend frequent fundoscopic exams under anesthesia in affected young children, uncertainty remains whether its risk–benefit ratio is similar for our unaffected twin considering her chimerism for the RB1 pathogenic variant.
Studies show that chimeric cells can last through adulthood (Farber et al., 1989; Sudik, Jakubiczka, Nawroth, Gilberg, & Wieacker, 2001). Chen and colleagues reported a 2-year follow-up on a case of MC/DZ opposite-sex twins who were both found to have two distinct blood cell lines, with the female twin having 18% of XY cell line and the male twin having 30% of XX chimeric blood cells (Chen, Chmait, Vanderbilt, Wu, & Randolph, 2013). Chimerism was not noted in the analysis of buccal samples of these twins, suggesting that it is likely confined to the blood. Clinical assessment of these twins at 9 months of age was normal. Both twins had normal motor development, and except for mild weakness in expressive language skills, they both had a normal physical exam at 2 years old. The female twin had normal internal genital organs at 28 months of age. Interestingly, the percentage of opposite sex bloodlines at 28 months was higher than the initial report at 6 months of age in both twins (Chen et al., 2013).
The beneficial or adverse health prediction of chimerism and cancer susceptibility has been a topic of interest for many researchers. Mitchell and colleagues studied tumor tissue of 11 first degree phenocopies with ovarian, primary peritoneal and endometrial cancers in families with hereditary breast and ovarian cancer (HBOC) with a known pathogenic variant in BRCA1 or BRCA2. They speculated that these phenocopies who did not have the family variant in their blood might have had the chimeric cells in their affected tissues. However, they did not identify the familial variant in the tumor tissue of any of the phenocopies. Ultimately, their study did not support their hypothesis (Mitchell et al., 2018).
Another study that can shed light on the clinical consequences of chimerism in humans is the study of fetal cell microchimerism. Fetal cells can persist in maternal blood and tissue for decades after they first appear in mothers (Bianchi, Zickwolf, Weil, Sylvester, & DeMaria, 1996). Nemescu and colleagues showed that the concentrations of male fetal chimeric cells are increased in cancer tissue of mothers with breast cancer in comparison with their unaffected breast tissue and blood (Nemescu, Ursu, Nemescu, & Negura, 2016). Cirello and colleagues also reviewed the protective and damaging effects of fetal cell chimerism in maternal cancers (Cirello & Fugazzola, 2016). These studies posit contrasting favorable and unfavorable effects of chimeric fetal cells on cancer in the affected mothers; however, the pathophysiology and accuracy of these predictions remain unknown.
Chimerism in monochorionic twins appears to be more common than previously thought. This likelihood is still high in MC/DZ twinning, a rare event, specifically in naturally conceived twins. However, the extent and potential clinical implications of chimerism, whether confined to the blood or not, have not been well studied, probably because many human chimeras remain undetected and the full extent of the chimerism is challenging to assess. The majority of reported cases of human chimerism in MC/DZ twins have been able to identify chimeric cells only in blood. There are at least two reports of MC/DZ twins with chimerism in cells other than blood (Fumoto et al., 2014; Rodriguez-Buritica, Rojnueangnit, Messiaen, Mikhail, & Robin, 2015). Fumoto and colleagues raised the possibility of cryptic chimerism in other organs and possibly gonads in addition to blood. Unfortunately, we were unable to analyze more tissues such as buccal samples from the twins in the clinical setting. These findings pose the complexity of proper cancer risk assessment in the unaffected twin in our case and the risk of transmission of the RB1 variant to her offspring.
In our twins, chimerism was not noted in the analysis of fibroblast samples. All assessed SNPS indicated allele fractions consistent with homozygosity or heterozygosity for the SNP calling/position. Heterozygous levels of the RB1 variant in twin A, with bilateral retinoblastoma, suggest this variant arose very early in development or is actually constitutional in this twin, as a result of germline mosaicism in one of the parents. The apparently equal and persistent mixing of zygosity of variants in the blood suggests shared blood supply and possibly stem cells during gestational development (Figure 2).
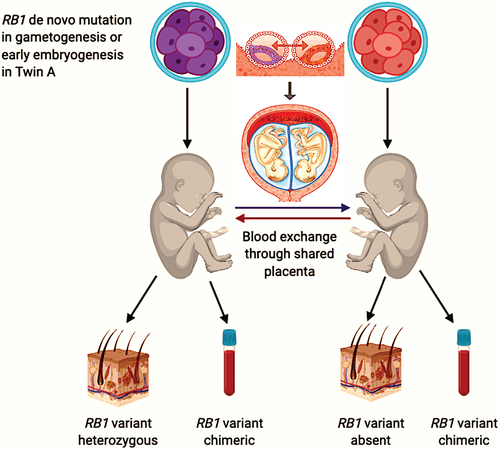
To date, the unaffected twin has not developed retinoblastoma. However, given the unpredictability described above, we do not plan to stop surveillance for retinoblastoma in her. Uncertainty remains regarding cancer risk in the unaffected twin and her offspring. Long-term follow-up of our twins may provide helpful information on the phenotypic consequences of human chimerism.
4 CONCLUSION
The formation of natural human chimeras appears to be a more common phenomenon than previously thought. Despite the long discovery of human chimeras, the potential clinical implications of chimerism have not been well studied. Long-term follow-up of our twins and additional new cases as awareness increases will provide information that can help elucidate this area of ambiguity.
ACKNOWLEDGMENTS
We would like to thank the twins' parents and also would like to extend our gratitude to GeneDx genetic laboratory for facilitating genetic testing for these twins. Authors Anna M. Armitage, Monica A. Kundra, Neda Ghiam, Paldeep S. Atwal, Dayna Morel, and Irman Forghani have no disclosures. Rebecca Torene and Kathleen S. Hruska are employees of GeneDx, Inc., a wholly owned subsidiary of OPKO Health, Inc.
AUTHOR CONTRIBUTIONS
Anna M. Armitage and Monica A. Kundra have prepared the original draft. Neda Ghiam has prepared and submitted the manuscript. Paldeep S. Atwal has reviewed and edited the manuscript. Dayna Morel has reviewed and edited the manuscript and managed the patients. Kathleen S. Hruska and Rebecca Torene have reviewed and edited the manuscript and developed diagnostic definitions. Irman Forghani has supervised the study and manuscript development, performed patient management and diagnostic definition.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




