An apparent new syndrome of extreme short stature, microcephaly, dysmorphic faces, intellectual disability, and a bone dysplasia of unknown etiology
Abstract
We report on a 26-year-old male with extreme short stature, microcephaly, macroglossia, other dysmorphic features, severe intellectual disability, and a bone dysplasia. The patient had an extensive genetic and biochemical evaluation that was all normal or noninformative. Recently, the proband died following a period of not eating. He likely had a previously undescribed syndrome of unknown etiology.
1 INTRODUCTION
There are over 450 recognized types of heritable skeletal dysplasias (Mortier et al., 2019). Typically, a combination of clinical evaluation, radiographic imaging, and molecular testing is employed to reach a diagnosis of these conditions. Here we present a patient who had a distinct phenotype of unknown etiology despite extensive evaluations.
2 CLINICAL REPORT
We present a 26-year-old Caucasian male whom we first evaluated as an infant, then later at ages 20 and 25 years. His major findings included extreme short stature, microcephaly, macroglossia, other dysmorphic features, an apparent unique bone dysplasia, developmental delay, and severe intellectual disability. He was born at 40 weeks gestation following a pregnancy complicated by intrauterine growth restriction. His birth weight was 2.7 kg, (10th percentile), length was 44.4 cm (−2.5 SD), and occipitofrontal circumference (OFC) was 28 cm (−3.5 SD). As an infant, the patient developed hydrocephalus that required ventriculoperitoneal shunting. Macroglossia developed during infancy and at age 12 years, he had surgical tongue reduction. At this time, there is no available pathology report on the tongue. He also had severe growth delay and at age 25, he was 73 cm tall (−10.3 SD), weighed 14.6 kg (−4.8 SD) and had an OFC of 40 cm (−10.2 SD). In addition, the patient had a history of severe global developmental delay, and significant intellectual disability, and as an adult, an estimated developmental age of 2 years.
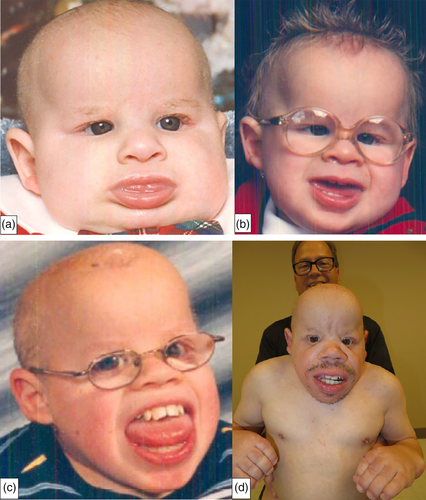
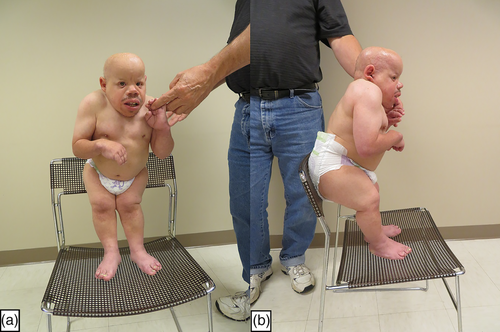
Other clinical findings included coarse facial features (Figure 1a–d); dolichocephaly; sparse and brittle hair; a prominent forehead; a salt and pepper retinopathy with abnormal maculas, microcorneas, and hyperopia; flat nasal bridge; midface hypoplasia; and prominent jaws. Although he had abnormal eye findings, he never developed cataracts. In addition, he also had small ear canals, bilateral conductive hearing loss, sparse and brittle hair especially of the scalp, and a generalized follicular rash. There also was a distinct body habitus with short arms, legs, and trunk; contractures (Figure 2a,b); prominent muscles; and very little subcutaneous fat. His hearing deficit was first found at the age of 3 years when he had auditory brainstem response (ABR) testing using clicking. His threshold then was 35 dB on the right and 45 dB on the left. At age 20, he was diagnosed with severe hearing loss with no responses to ABR greater than 80 dB at frequencies between 250 Hz to 4,000 Hz.
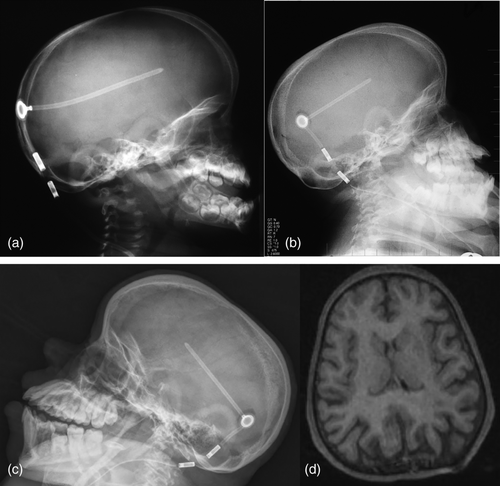
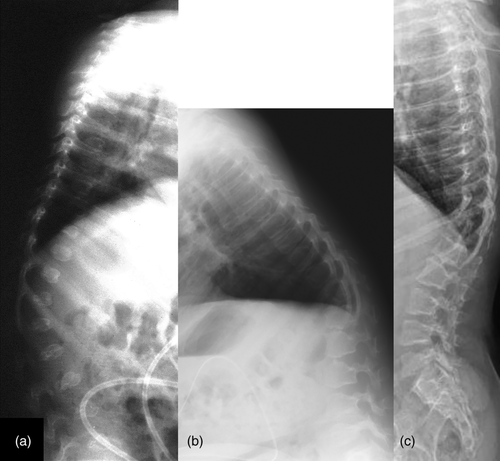
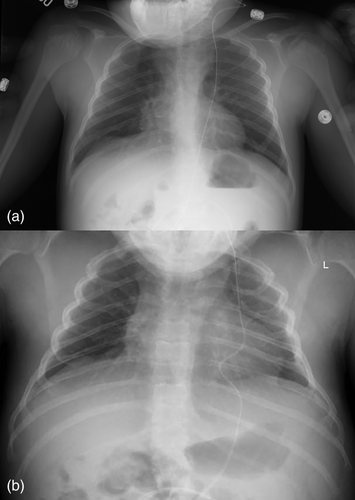
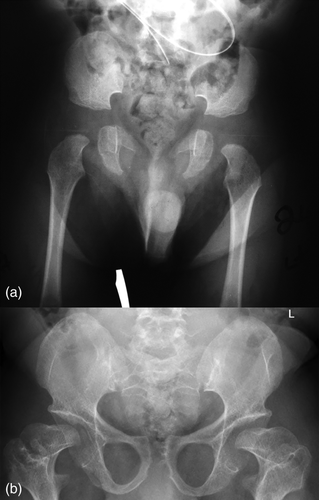
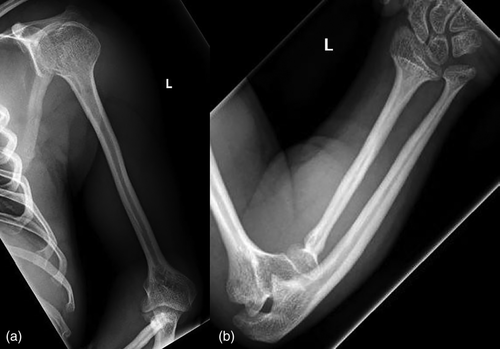
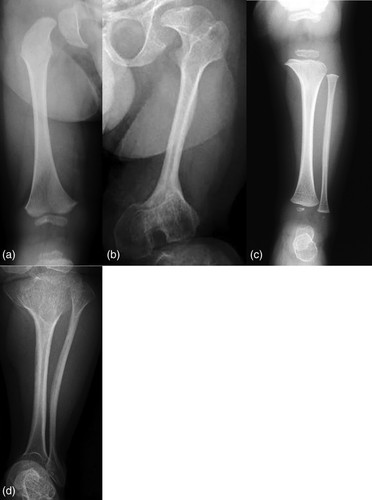
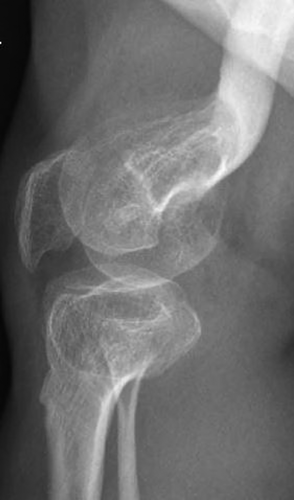
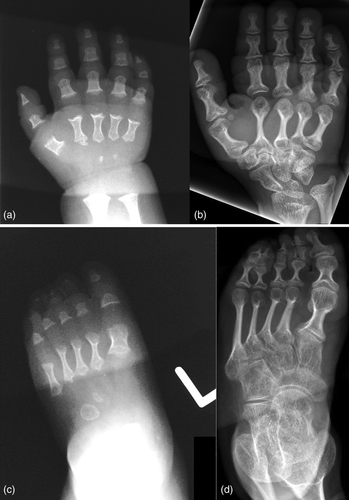
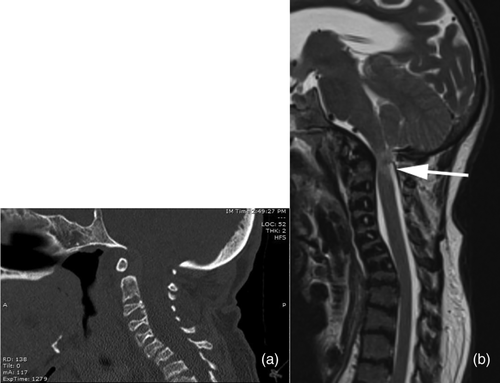
Radiographically, he had an apparent unique bone dysplasia as depicted in skeletal surveys at ages 12 and 17 months, and 20 and 25 years (Figures 3-11). The earlier radiographs show most of the same findings as the later ones except the findings are not as marked (Figures 3a,b, 4a,b, 5a, 6a, 7a,b, 8a,c, and 10a,c). The latter radiographs show shortened and thinness of the diaphyses of the long bones, metacarpals, metatarsals, and phalanges with progressive enlargement of the metaphyses and epiphyses, (Figures 7c,d, 8b,d, and 10b,d); proximal ring-like narrowing of the radial diaphysis (Figure 7d); excessive slanting of the distal metaphyses of the ulna and fibula (Figures 7d and 8d), marked notching of the distal femur (Figure 8b) and overgrowth of the fibulas distally in relation to the tibias (Figure 8d). Other skeletal findings include narrow and oval-shaped pelvic inlet, no sciatic notches, poorly formed acetabulums, markedly irregular flat femoral heads with short femoral necks (Figure 6b), 50° anterior angulation of the distal femoral metaphyses (Figure 9), and slender ribs (Figure 5a,b). Skull imaging at 12 months showed mild dolichocephaly with normal proportion of facial bones and skull (Figure 3a), and later, progressive elongation and enlargement of the jaws, thickened skull with a J-shaped sella turcica and platybasia (Figure 3b,c). Other than for the microcephaly, the brain magnetic resonance imaging (MRI) at age 20 showed normal structures with resolution of the hydrocephalus following shunt placement (Figure 3D). Spinal abnormalities include short pedicles, posterior wedging of all lumbar vertebral bodies, irregular vertebral body endplates, a thoracolumbar gibbus secondary to anterior wedging of T12, reduced height of the cervical spine vertebrae, non-ossified odontoid, fused C2–C3 posterior vertebral spines and facets with severe narrowing at the craniocervical junction resulting in secondary spinal cord myelomalacia (Figures 4a–c and 11a,b). He also had irregular, punctate calcifications of the trachea. A chest radiograph at the age 12 years demonstrate irregularities and broadened proximal humoral metaphyses; possibly thin proximal humoral epiphyses; thin humoral diaphyses, clavicles and ribs; 11 ribs bilaterally, and small chest size (Figure 5a,b). Note that in Figure 5a, the proximal humeral growth plates are still open.
Laboratory evaluation of the proband included a karyotype, chromosomal microarray, urine biochemical screening with mucopolysaccharide spot, a serum growth hormone stimulation study, very long chain fatty acids, copper and ceruloplasmin levels, complete blood count, comprehensive metabolic panel, creatine kinase level, thyroid stimulating hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 levels, and hair pathology including light polarization. All of the above evaluations were normal. Serum lysosomal enzymes were also normal; however, global metabolomics analyses returned with many analytes significantly altered, suggesting a potential dysfunction of peroxisome biogenesis but no specific diagnosis. From radiographic assessment of the proband's skeletal surveys at ages 20 and 25, the UCLA International Skeletal Dysplasia Registry did not recognize a specific syndrome.
In 2014, trio whole exome sequencing (WES) (GeneDx, Gaithersburg, MD) was performed on the patient and his parents, which revealed three variants. The first was a variant of uncertain significance (VUS), c.*32 + 1G>A, in the PTPN11 gene. His father has the same variant and has no identified exostoses or heart defects. The proband had no exostoses (Figures 6a,b) or heart defects, either. A heterozygous pathogenic variant, c.304C>T (p.R102X), was identified in the MCOLN1 gene. His mother also carries this mutation. The final variant was a heterozygous VUS, c.1294G>C (p.D432H), in the ZNF750 gene, which the mother also carries. She has none of the symptoms associated with mutations in this gene. Upon reanalysis of the proband's WES (GeneDx) in August 2018, a de novo VUS, c.1279G>C (p.G427R), was reported in the GPKOW gene, which is located on the X chromosome.
Trio WGS were also completed. These studies also found the de novo a VUS, c.1279G>C (p.G427R) in the GPKOW gene. In addition, a hemizygous variant in DKC1 (c.-142C>G) of maternal inheritance and a heterozygous DIAPH3 variant (c.357G>C) of paternal inheritance were found. Both of these variants were also classified as VUSs. The WGS report (Illumina's TruGenome Undiagnosed Disease Test) did not report the variants PTPN11, MCOLN1, and ZNF750 cited in the WES study.
The proband had no family history of birth defects or bone dysplasias. His father and his maternal grandfather both have had progressive hearing loss that began in childhood. The etiology of this hearing loss is unknown. Consanguinity was denied. He had no siblings.
When we reevaluated the patient at the age of 25, there were no significant changes in his phenotype from age 20, except for a progressive decrease in mobility. Head MRI at the time revealed microcephaly, a left parietal venticulostomy catheter with collapse of the lateral ventricles, hypoplastic or absence of the internal auditory canals and an abnormal contour of the pituitary gland without focal lesions. Spinal computed tomography (CT) and MRI imaging subsequently revealed deformities at the craniocervical junction resulting in spinal cord compression and myelomalacia (Figure 11a,b). Treatment for the spinal cord compression consisted of bracing. Surgical and other interventions were considered but were not undertaken. At the age of 26, the proband died peacefully after not eating for 3 weeks.
3 DISCUSSION
The patient reported here appears to have had a previously undescribed dysmorphic syndrome. Despite an extensive evaluation, we have not been able to identify an etiology. The most distinct features of his syndrome include his distinctive facial appearance, microcephaly, macroglossia, unique skeletal changes, severe short stature, and severe intellectual disability.
We have considered various differentials for the patient's condition. These primary conditions include primordial dwarfisms (microcephalic osteodysplastic primordial dwarfism Types I/III and II (MOPD) and Seckel, Russell-Silver and Meier-Gorlin syndromes (Bober & Jackson, 2017; de Munnik et al., 2015; Khetarpal, Das, Panigrahi, & Munshi, 2016; Spiteri, Stafrace, & Calleja-Agius, 2017; Yigit et al., 2015), spondyloepimetaphyseal dysplasias including spondyloepimetaphyseal dysplasia X-linked biglycan related and spondyloepimetaphyseal dysplasia X-linked with hypomyelinating leukodystrophy (SEMDXL) (Bieganski, Dawydzik, & Kozlowski, 1999; Meester et al., 2017; Miyake et al., 2017; Tiller et al., 1995), metatropic dysplasia (Gucev et al., 2018), cartilage hair hypoplasia (Mäkitie, Sulisalo, de la Chapella, & Kaitila, 1995), anauxetic dysplasia (Akgun-Dogan, Simsek-Kiper, Utine, & Boduroglu, 2018), Nicolaides-Baraiter syndrome (Sousa et al., 2009), disorders of peroxisome biogenesis including Zellweger spectrum disorders and rhizomelic chondrodysplasia punctata type 1 (Argyriou, D'Agostino, & Braverman, 2016; Braverman et al., 2016), and storage diseases. We also have considered Marshall–Smith syndrome (MSS) in the differential diagnosis as some published cases with this condition have similar facial appearance to the patient here. However, the natural history and the radiographic findings, particularly in the hands, are considerably different in MSS (Shaw et al., 2010). Growth retardation alopecia pseudoanodontia aptic atrophy (GAPO) syndrome is another consideration in the differential. The disorder is characterized by a complex of growth retardation, alopecia, failure of tooth eruption, and progressive optic atrophy (Tipton & Gorlin, 1984) and is an autosomal recessive condition caused by variants in the ANTXR1 gene (Bayram et al., 2014). Although in each of the above-mentioned conditions there are overlapping features with our patient, he does not totally match any of them, we found no biochemical alterations in him associated with these disorders and/or he had no mutations in any of the genes that cause these conditions.
With regard to the variants found in our patient's WES, the VUS c.+32+1G>A change in the PTPN11 gene is associated with PTPN11-related disorders (LEOPARD syndrome 1, metachondromatosis, Noonan syndrome, and juvenile myelomonocytic leukemia) (Tartaglia et al., 2001; Tartaglia et al., 2003). Our patient clearly does not have any of these conditions. The single pathogenic mutation in the MCOLN1 gene c.304C>T (p.R102X), when inherited in a homozygous fashion, causes mucolipidosis type 4 (Bargal et al., 2000). Typically, mucolipidosis type 4 is not associated with a bone dysplasia. Additionally, our proband only has one MCOLN1 variant. The final VUS in the ZNF750 gene, c.1294G>C (p.D432H), is associated with an autosomal dominant form of seborrheic dermatitis with psoriasiform elements (Birnbaum et al., 2006). The patient's rash had been successfully treated with antifungal cream and the VUS in this gene does not explain his additional features.
We have also considered the possibility of the de novo VUS in the GPKOW gene reported both in the patient's WES and WGS as contributing to or causing our proband's condition. The GPKOW gene encodes for the GPKOW protein, which functions in human spliceosomes and has been shown to be important for pre-mRNA splicing (Zang et al., 2014). Other spliceosome inactivation has been shown to cause isolated microcephaly and microcephalic osteodysplastic primordial dwarfism type I (Baumgartner et al., 2018). In one report, GPKOW is reported to be critical for intrauterine growth and development, and mutations in this gene can cause severe microcephaly and intrauterine growth restriction, and is lethal in males (Carroll et al., 2017). The variant reported in our patient, c.1279G>C (p.G427R), has not been previously reported, has not been reported in any large cohorts and is a nonconservative substitution (Lek et al., 2016). In silico models predict this c.1279G>C mutation has a deleterious effect on the GPKOW protein. However, this prediction is not sufficient to definitively state that the mutation was causative in our patient. On the other hand, this variant could have contributed to his phenotype.
The two VUSs reported from our patient's WGS were c.-142C>G in DKC1 and c.357G>C (p.Glu119Asp) in DIAPH3 and reported to be of unknown significance (Illumina Inc., San Diego, CA). DKC1 is inherited in an X-linked fashion and mutations in the gene can cause dyskeratosis congenita (DC). Characteristics of this latter condition include the triad of dysplastic nails, lacy reticular pigmentation of the skin, and oral leukoplakia (Ballew & Savage, 2013; Heiss et al., 1998). Neither the patient nor his mother, who carries this variant, had/has any of these features. There also is a variant in DKC1 that produces the Hoyeraal–Hreidarsson syndrome (HHS), a more severe form of DC. This condition includes the above triad plus intrauterine growth deficiency, early onset developmental delay, cerebellar hypoplasia, immunodeficiency, and bone marrow failure (Ballew & Savage, 2013; Heiss et al., 1998). Other than for developmental delay, the proband had none of these features and was unlikely to have had HHS. Variants in DIAPH3 are associated with absent or abnormal auditory brainstem responses in the presence of normal cochlear outer hair cell function and normal otoacoustic emissions (Ballew & Savage, 2013; Heiss et al., 1998). There is limited information on this c.357G>C variant, and in part then, is why it was classified as a VUS. Since mutations in DIAPH3 are associated with hearing loss, it is possible that our patient's and his father's hearing loss was/is caused by the mutation in this gene.
Untargeted global metabolomic pathway screening is a way to look for perturbations in individual biochemical pathways. This testing utilizes mass-spectrometry methods to assist in detection of known and unknown inborn errors of metabolism, and has been shown to be of diagnostic assistance (Miller et al., 2015). Specifically, the test used for our patient detects compounds of 50–1,500 Da sizes. Grossly abnormal analytes in our patient testing (Supplemental Appendix S1) suggested a potential disorder of peroxisome biogenesis but not for a specific disorder. Significantly, our patient does not have elevated very long chain fatty acid levels, flat occipital bone, ocular abnormalities nor hepatomegaly that are typically observed in disorders of peroxisome biogenesis (Braverman et al., 2016). Our proband also did not have cataracts, punctate calcifications, or variants in the PEX genes. Furthermore, disorders of peroxisome biogenesis typically do not have the marked bone dysplasia and microcephaly observed in our patient.
Typically, peroxisomal disorders fall into two categories: Zellweger spectrum disorder and rhizomelic chondrodysplasia punctata type 1 (Argyriou et al., 2016). Zellweger spectrum disorder characteristically presents with flat occipital bone, ocular abnormalities, hepatomegaly, and elevated very long chain fatty acid levels, and is caused by mutations in the PEX genes (Braverman et al., 2016). Rhizomelic chondrodysplasia punctata type 1 is characterized by short humeri and femurs, punctata calcifications, cataracts, characteristic clefting of the vertebral bodies and pathogenic mutations in PEX7 (Argyriou et al., 2016). Our patient did not appear to have either condition.
The authors recognize that the patient reported here may not have had a new dysmorphic disorder but could well have had a different expression of a recognized condition that we have not identified. The disorder could have been of a genetic origin and we were not be able to identify the nefarious gene or chromosome abnormality with the genetic testing done.
ACKNOWLEDGMENTS
We would like to thank the patient and his kind parents for their cooperation and enthusiasm that made this project possible. The authors obtained signed consent from the parents to publish information and photos of the proband. We would also like to thank additional contributors to the patient's workup and completion of the project including Katie Sapp, Kate Burns, Dr. Erik Imel, Dr. Andrew Jea, Dr. Ralph S. Lachman of the International Skeletal Dysplasia Registry, and Illumina iHope program. This article is dedicated to the proband.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
AUTHOR CONTRIBUTIONS
J.B., B.H., and D.W. contributed to the patient's clinical evaluation both in infancy and as an adult. J.S. and D.W. lead the manuscript preparation and revisions. J.B. contributed to the Abstract and Clinical Report, and B.K. did the radiographic analyses, wrote the radiographic discussion and legends, and prepared the radiographic photos. Brett Graham assisted in metabolic work up and analyses and in creating the supplemental material. All authors read, contributed to revisions and approved the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




