CDH1-related blepharocheilodontic syndrome is associated with diffuse gastric cancer risk
Abstract
We report the first case of diffuse gastric cancer in an individual with familial blepharocheilodontic syndrome (BCD) due to a germline CDH1 likely pathogenic variant. To date, other BCD affected relatives are nonpenetrant for diffuse gastric cancer posing challenges to counseling regarding gastric and breast cancer surveillance, and preventative total gastrectomy.
1 BLEPHAROCHEILODONTIC SYNDROME AND DIFFUSE GASTRIC CANCER RISK
Blepharocheilodontic syndrome (BCD; OMIM 119580) is a rare disorder characterized by eyelid malformations, cleft lip and palate (CLP) and dental anomalies (Gorlin et al., 1996). The most consistent feature is CLP, which is most often bilateral. Eyelid malformations include ectropion of the lower eyelids, euryblepharon (horizontal enlargement of the palpebral fissure), lagophthalmia (inability to completely close the eyelids), and distichiasis (double row of eyelashes; Kievit et al., 2018). Variable features of ectodermal dysplasia have also been described, particularly involving the teeth (conical teeth and tooth agenesis). Less commonly reported features include hypothyroidism due to thyroid gland hypoplasia or aplasia, imperforate anus, neural tube defect, and syndactyly (Ababneh, Al-Swaid, Elhag, Youssef, & Alsaif, 2014; Weaver, Rutledge, Grant, & Robin, 2010). While most cases of BCD are isolated, large affected families have been described, with pedigrees consistent with autosomal dominant inheritance (Ababneh et al., 2014; Gorlin et al., 1996; Gorlin & Wiedemann, 1996; Guion-Almeida, Rodini, Kokitsu-Nakata, & Bologna-Amantini, 1998; Iiada, Narai, Takagi, Ono, & Ikenda, 2006; Weaver et al., 2010). CDH1 missense variants and CTNND1 truncating variants have been identified in families with BCD (Ghoumid et al., 2017).
CDH1 variants were first identified in families with dominantly inherited diffuse gastric cancer (DGC) (Guilford et al., 1998). More recently, it was recognized that the cancer phenotype of hereditary DGC (HDGC; OMIM 137215) due to CDH1 variants includes lobular breast cancer (LBC) (Hansford et al., 2015; van der Post et al., 2015). CDH1 variants have also been reported in nonsyndromic CLP (NSCLP) without cancer, as well as in families with both NSCLP and HDGC (Benusiglio et al., 2013; Brito et al., 2015; Frebourg et al., 2006; Huynh & Laukaitis, 2016; Kluijt et al., 2012; Vogelaar et al., 2013). DGC and LBC have not been reported previously in patients with BCD. Here we report the first such family.
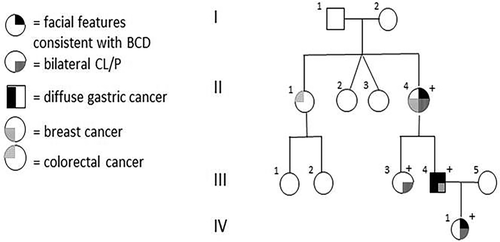
The proband (III.4), a 33-year-old male with repaired bilateral cleft lip and palate, oligodontia, and dysmorphic features (Figure 1) was seen in 2012 for genetic counseling. There was a family history of bilateral CLP and oligodontia in his mother and sister (Figure 2). No syndromic diagnosis was recognized at this time and counseling was based on the pedigree, which was in keeping with autosomal dominant inheritance. The proband, his mother, and his sister were enrolled in a gene discovery project in 2013. Whole exome sequencing performed at the University of Washington Genome Centre identified missense variant in CDH1 (c.768 T > A, p.N256K). This variant was classified as likely pathogenic by the reporting laboratory (January 2014).
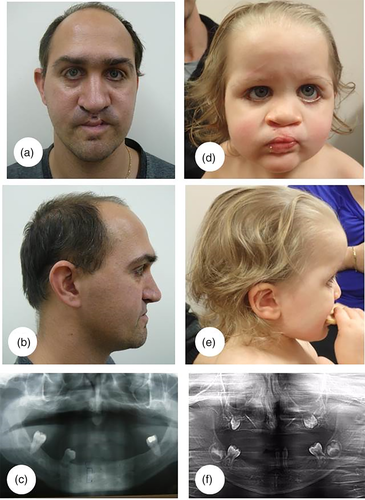
The proband's daughter (IV.1), now aged 6 years, was born in mid-2014 at 39 weeks gestation after a pregnancy in which bilateral CLP was diagnosed on second trimester morphology ultrasound. Testing shortly after birth confirmed that she had the familial CDH1 variant. She had similar facial features to her affected relatives, now recognized as consistent with BCD (Figure 1), and additional features not seen in other family members. Newborn screening showed elevated TSH levels and subsequent thyroid nuclear scan confirmed thyroid agenesis. An echocardiogram was performed because of a cardiac murmur and branch pulmonary stenosis was identified. Cleft lip was repaired at 2 months of age followed by palate repair at 10 months of age. Mild tracheobronchomalacia and subglottic stenosis resulted in obstructed breathing following palate repair. She had a low anterior hairline with unusual frontal hairline whorls, high forehead and flat midface, bilateral ectropion and left epidermoid cyst. An orthopantomogram at 3 years 7 months old showed marked thinning of maxillary alveolar bone and a relatively edentulous maxilla and mandible. The only radiologically visible teeth were a molar tooth on each side of the maxilla, sevens and eights in the mandible and a central incisor and canine in the left hemi mandible (Figure 1).
CT scan of the orbits and petrous temporal bones revealed bilateral enlargement of the vestibular aqueduct, along with the clinically identified left epidermoid cyst and a similar nonpalpable lesion on the right. Given the association between vestibular aqueduct enlargement and Pendred syndrome, sequencing of SLC26A4 was performed; no variant was identified. To our knowledge, enlargement of the vestibular aqueduct has not been previously reported in BCD.
Segregation testing showed all family members with CLP carry the CDH1 c.768 T > A variant. The family was reported as CDH1-associated nonsyndromic CLP (Cox et al. 2018).
The proband (III.4) was diagnosed with symptomatic advanced diffuse gastric cancer at 37 years of age. His mother (II.4) was diagnosed with breast cancer at age 48 years (infiltrating ductal carcinoma with a component of invasive lobular carcinoma) and adenocarcinoma of the lung at age 51 years. His maternal aunt (II.1) died of colorectal cancer at the age of 63 but had no features of BCD. Following the proband's diagnosis with DGC, his 63-year-old mother and 34-year-old sister had gastric endoscopy. Multiple random biopsies of the gastric mucosa of both individuals did not detect any foci of in situ signet ring cells or invasive DGC. Given her other health issues, his mother declined preventative total gastrectomy (PTG) but has had normal endoscopic surveillance to date. His sister is having endoscopic surveillance 6 monthly while considering the potential risks and benefits of PTG.
Variant classification was reviewed (Lee et al., 2018; Richards et al., 2015). CDH1 c.768 T > A is not listed in ClinVar. The variant has been reported in at least one additional family with BCD (Kievit et al., 2018) but has not been reported in association with HDGC. Using CDH1-specific ACMG guidelines, the variant is classified as a variant of unknown significance (VUS) against a DGC background because of lack of functional studies assessing the effect on cancer proliferation and only one generation of segregation with HGDC (Lee et al., 2018). It is classified likely pathogenic against a BCDS background, taking into consideration the functional studies of Kievit et al., (2018) and segregation with disease across three generations (Lee et al., 2018).
The CDH1 gene maps to chromosome 16q22.1 and encodes the cell-to-cell adhesion protein E-cadherin (Masciari et al., 2007). E-cadherin is highly expressed in human and mouse embryos during critical stages of lip and palate development (Frebourg et al., 2006; Montenegro, Rojas, Dominguez, & Vergara, 2000). Several mouse models confirm the critical role of E-cadherin for eyelid, craniofacial, tooth, and hair development (Bartlett et al., 2010; Li et al., 2012; Shimizu et al., 2005; Smith, Dohn, Brown, & Reynolds, 2012).
Germline CDH1 variants are implicated in both NSCLP and BCD (Benusiglio et al., 2013; Brito et al., 2015; Frebourg et al., 2006; Ghoumid et al., 2017; Kluijt et al., 2012; Vogelaar et al., 2013). Frebourg et al. (2006) reported cleft lip (CL) or cleft palate (CP) in five CDH1 variant carriers from two families (Frebourg et al., 2006). Kluijt et al. (2012) reported similar findings in four CDH1 variant carriers from two distinct families with HDGC (Kluijt et al., 2012). In 2013, Benusiglio et al. described a 17-year-old girl of Southeast Asian origin with a history of repaired cleft lip and palate, who was also found to have a CDH1 variant when investigations for iron deficiency anemia led to a diagnosis of DGC (Benusiglio et al., 2013).
HDGC (OMIM #137215) is an autosomal dominant cancer predisposition syndrome with high penetrance. Depending on the case definition used, germline CDH1 variants are identified in 15–50% of kindreds with HDGC (Huynh & Laukaitis, 2016; Kluijt et al., 2012; van der Post et al., 2015). To date, more than 180 different germline CDH1 variants have been identified in HDGC families in a diverse range of ethnic groups. In 2015 the International Gastric Cancer Linkage Consortium (IGCLC) published consensus guidelines for CDH1 germline testing and gastric cancer risk management (van der Post et al., 2015). A recent update to these guidelines was generated after the IGCLC meeting held in early 2019 and publication is pending (personal communication Dr P Guilford, Dr V Blair 12/03/2020).
For CDH1 variant carriers ascertained via a family history of DGC, the cumulative risk of DGC at age 80 years is 70% for men and 56% for women (Huynh & Laukaitis, 2016). There is also a high risk of LBC in females (42% to age 80 years) (Corso et al., 2014; Hallowell et al., 2016; Hansford et al., 2015). DGC is usually asymptomatic until an advanced stage, when it has poor prognosis (5 year survival <30%). PTG is the only way to prevent DGC and is recommended for all asymptomatic carriers of CDH1 pathogenic variants with a family history of DGC, despite gastrectomy having a significant impact on quality of life and increasing the risk of weight loss and malnutrition. The consensus is to recommend PTG in early adulthood, usually between the ages of 20 and 30 years (van der Post et al., 2015; Monahan & H. L., 2016; personal communication Dr P Guilford, Dr V Blair 12/03/2020). In HDGC families where DGC has occurred in teenage years or the early twenties, it may be appropriate to consider gastrectomy before age 20 years, ideally after the majority of adolescent growth is complete, but guidelines for this setting have not been published.
There are no formal guidelines for breast cancer surveillance in CDH1 variant carriers. Broad expert opinion supports breast MRI beginning at age 30 (Corso et al., 2018). Where there is a family history of lobular breast cancer some women consider risk reducing mastectomy, but this is not routinely recommended for CDH1 mutation carriers.
In addition to identifying previously unreported clinical features in a patient with BCD, our case is the first reported in which members of a family with BCD have also developed DGC or breast cancer with a lobular component. This case highlights the need for appropriate counseling regarding cancer risk in families with BCD. However, counseling is challenging as gastric and breast cancer penetrance in families ascertained via a BCD phenotype is unknown. There is a need for international collaboration to clarify the cancer risk in BCD and the optimal management of this risk.
1.1 EDITORIAL POLICIES AND ETHICAL CONSIDERATIONS
Written informed consent for publication of clinical information and photographs was obtained from each individual and/or their parent/guardian. The study was approved by the appropriate medical ethics committee.
2 CONFLICT OF INTEREST
We confirm that this manuscript has not been submitted elsewhere for publication. We certify that all co-authors have read the final manuscript and take responsibility for it and accept its conclusions. The authors do not have any conflict of interest, financial or potential conflict of interest to disclose.
AUTHORS’ CONTRIBUTION
Shannon LeBlanc, Dildeepa Naveen, Nicola Poplawski, Eric Haan, and Christopher Barnett contributed to drafting and revising the manuscript content. Tony Roscioli made substantial contribution with regards to identification and classification of the CDH1 gene variant identified in this family. Lesley Rawlings subsequently reviewed the classification of the variant with regards to CDH1 specific ACMG guidelines. All authors have given final approval of the version of the manuscript to be published.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




