Increased T-cell counts in patients with 22q11.2 deletion syndrome who have anxiety
To the Editor
22q11.2 deletion syndrome (22q11.2del) is the most common chromosomal microdeletion disorder, affecting 1 in 2,000–4,000 live births. It can be associated with developmental alterations including cardiac, palatal, immunologic, endocrine, and brain involvement, manifesting as developmental delays, cognitive deficits, and neuropsychiatric illnesses (Miller, Gassama, Sebastian, Buckley, & Mellor, 2013). Many studies have shown that 22q11.2del is the strongest known genetic risk factor for the development of psychosis, with ~25% of adults with 22q11.2del having a psychotic disorder (Monks et al., 2014; Murphy, Jones, & Owen, 1999; Schneider et al., 2014). Schizophrenia spectrum disorders (SSD) evolve by early adulthood and high rates of attention deficit hyperactivity disorder (ADHD), anxiety disorders, mood disorders and autism spectrum disorder (ASD) can be seen in children and may represent risk factors for future psychosis (Antshel et al., 2007; Schneider et al., 2014).
Emerging evidence supports a role for immune mechanisms in SSD. Some studies have found that T-cell-mediated immune responses were reduced in acute schizophrenia (Achiron et al., 1994; Steiner et al., 2010). Inflammatory activation of monocytes, macrophages, and microglia have also been described (Leza et al., 2015; Miller et al., 2013). Due to the well-characterized immunodeficiency and high risk for the development of psychosis, 22q11.2del can be an informative model for the study of mechanisms of inflammation in psychosis. In this study, we retrospectively analyzed the lymphocyte subsets in 159 patients with confirmed 22q11.2del, who were also participants in a study of brain and behavior in 22q11.2del. This study was approved by the Institutional Review Board at The Children's Hospital of Philadelphia. Flow cytometry was performed on all patients as part of their clinical assessment and was not synchronized with the psychiatric interview that was part of a prospective study, Brain-Behavior and Genetic Studies of the 22q11DS, at the University of Pennsylvania and Children's Hospital of Philadelphia (CHOP) (Tang & Gur, 2018). The average delay between immunologic evaluation and psychiatric evaluation was 7 years. Detailed methods are available in Data S1.
This group of patients with 22q11.2del included 86 males and 73 females. Among the 159 patients, 37 patients were <5 years old at the time of immunologic analysis, 33 patients were 5–10, 64 patients were 10–20, and 25 patients were >20 years old. Thirty-six patients (22.6%) had no psychotic symptoms, and 123 patients (77.4%) had a range of subthreshold psychotic symptoms (Table S1). The male/female ratio was comparable between all groups except ADHD where males predominated (p = .038), as is typical. We analyzed the absolute count of CD3, CD4, and CD8 T cells as cell types implicated in the immunodeficiency and considered relevant for inflammation. We additionally analyzed CD19 B cells as a control group unlikely to be altered in 22q11.2del. These were defined in different psychiatric symptom groups. Lymphocyte counts differ according to age, and therefore patients were divided into four groups according to the age at the time of study. MANOVA was performed to compare absolute counts of lymphocyte subsets between different age groups and whether there were psychiatric symptoms (Table S2). There was no statistically significant difference in CD3, CD4, CD8, and CD19 cells between all groups.
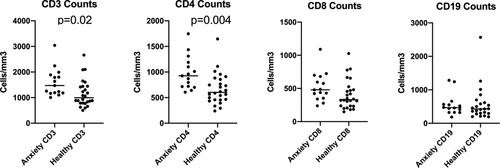
Generally, the mean absolute counts of CD3, CD4, CD8, and CD19 in patients in each affected group were lower than in patients without symptoms. In contrast, we noted that the absolute counts of CD3, CD4, CD8, and CD19 in patients with anxiety were higher than that in patients without anxiety, the opposite of what was seen for all other diagnostic categories. This prompted additional analyses. Among the 159 patients with 22q11.2del, 80 patients had anxiety disorders and 36 patients had no psychiatric diagnosis. Of the 80 patients with anxiety disorders, 22 had only anxiety, while 27 had comorbid ADHD, 42 had comorbid mood disorders, 10 had comorbid depressive disorders, 15 had comorbid psychosis spectrum disorders, and no patient had comorbid autistic spectrum disorder. We hypothesized that multiple diagnoses might alter the results since on the univariate analysis, anxiety behaved differently than the other diagnoses. We therefore analyzed the absolute counts of CD3, CD4, CD8, and CD19 cells in patients with 22q11.2del between 5 and 20 years of age with no psychiatric diagnosis (n = 26) and patients with anxiety disorder (n = 15) as an isolated diagnosis using a t test. CD3 and CD4 counts were significantly different between patients with anxiety disorder alone and no psychiatric diagnosis shown in Figure 1p ( = .02 and .004, respectively). There was no significant difference in age distribution between the two groups. CD8 and CD19 were not significantly different between the two groups. These additional analyses demonstrate that isolated anxiety disorder is associated with higher CD3 and CD4 T-cell counts.
22q11.2del is a primary immunodeficiency, characterized by T-cell deficiency in childhood related to thymic hypoplasia. The immunodeficiency evolves with age (Crowley, Ruffner, McDonald McGinn, & Sullivan, 2018; Morsheimer, Brown Whitehorn, Heimall, & Sullivan, 2017). The relationship of this well-characterized immunodeficiency to the high rate of psychosis is not understood and there have been few studies evaluating the relationship. Impaired T-cell function has been implicated in psychosis and T-cell dysfunction is the central feature of the immunodeficiency in 22q11.2del. Changes in T-cell cytokine production have been observed in SSD (Fineberg & Ellman, 2013). Potential mechanisms linking the two findings could be at the level of increased susceptibility to infection. Prenatal infections are risk factors for schizophrenia (Brown, 2011; Canetta & Brown, 2012). One of the central findings in 22q11.2del is the immunodeficiency which predisposes to infections and prolonged viral infections in particular. The immunodeficiency could lead to chronic inflammation that might alter the blood brain barrier. Indeed, increased serum cytokines have been observed in schizophrenia and in patients with 22q11.2del (Leza et al., 2015; Miller et al., 2013; Ross, Guo, Coleman, Ousley, & Miller, 2013). Furthermore, increased IL-17 producing T cells have been seen in patients with 22q11.2del who have psychosis (Vergaelen et al., 2018). Therefore, there are compelling reasons to try to understand the interaction of the immune system with brain and behavior. A recent study showed that 84% of the children with 22q11.2del had at least one psychiatric disorder (Serur et al., 2019). Thus, there is a great need to improve our understanding of this complication.
This study examined lymphocyte subsets in a large cohort of patients with 22q11.2del with and without psychiatric symptoms. Neither univariate nor multivariate analysis showed difference in T-cell subsets including CD3, CD4, and CD8 between the 22q11.2del with and without psychosis features. Instead, we found that the mean counts of CD3 and CD4 cells were higher in patients with anxiety than that in patients without any psychiatric diagnosis. As important producers of cytokines, T cells can influence the inflammatory response and play a role in the immune-brain communication. A previous study showed that patients with anxiety disorder had a markedly elevated ratio of CD4 versus CD8 lymphocytes compared to healthy controls and patients with minor depression (Atanackovic, Kroger, Serke, & Deter, 2004). The biology underlying the defective balance between CD4 T cells and CD8 T cells in anxiety disorders will require additional study. In mice, manipulation of T cells can improve anxiety-like behaviors suggesting that immunologic therapeutics may 1 day be possible for anxiety (Rattazzi et al., 2013). Murine studies have also demonstrated a key role for T cells in dendrite pruning, a process that may be implicated in psychosis (Acharjee et al., 2018; Terrell & Jordan, 2013). Although the mechanism is still unclear, our results suggest that CD3 and CD4 T cells may play an important role in the development of anxiety in patients with 22q11.2del.
This study represents the largest study to date of immunologic parameters in a carefully defined set of patients with behavioral and psychiatric symptoms. Nevertheless, there are limitations to this study. Lymphocyte subset analyses were not synchronized with the psychiatric evaluation. Thus, we may have underestimated associations if the immunologic parameters are dynamic. Many patients had multiple psychiatric diagnoses which again may have limited our ability to detect single outcome associations. Our data point out the critical need to address more formally associations between immunologic dysfunction and psychosis in this syndrome. Notably, our sample was young at intake into the study and still at risk for emergence of schizophrenia spectrum disorders. Longitudinal follow-up and concomitant immunologic measures can address this limitation.
In conclusion, 22q11.2del has a very high incidence of both behavioral symptoms and immunodeficiency, making it an ideal model for the study of psychoimmunology. Although there were no significant differences in the absolute count of CD3, CD4, CD8, and CD19 cells between patients with and without psychotic symptoms, we did find a strong association with anxiety. The CD3 and CD4 counts were significantly higher than that in patients without any symptoms. This suggested that CD3 and CD4 T cells may play an important role in the development of anxiety in 22q11.2del patients.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge our patients and clinical staff.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Ying Dou, T. Blaine Crowley, Sean Gallagher, Alice Bailey, Daniel McGinn, and Raquel E. Gur performed critical data analyses. Raquel Gur, Donna McDonald McGinn, and Elaine Zackai provided the clinical interpretation. Kathleen Sullivan wrote the first draft of the manuscript with Ying Dou which was subsequently edited by all involved.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




