Two middle-aged women with the Finnish variant of muscle-eye-brain disease (MEB)
Abstract
Muscle-eye-brain disease (MEB) is a recessively inherited rare disease. Sixteen different gene mutations are known, with the most common mutations in the POMGNT1 gene. The disease is now called congenital muscular dystrophy-dystroglycanopathy type A3 (MDDGA3). It manifests itself as muscular dystrophy with eye and brain anomalies and intellectual disability. Previous clinical reports describe young patients. We have been able to follow two patients for almost 40 years. Their clinical picture has remained quite stable since adolescence, appearing as severe intellectual and motor disability, extremely limited communication skills, visual impairment, epilepsy, joint contractures, repeated bowel obstructions, teeth abrasion due to bruxism, an irregular sleep pattern and as a previously unreported feature hypothermic periods manifesting as excessive sleepiness.
1 INTRODUCTION
People with an intellectual disability (ID) form the largest single disability group. There is either a genetic and/or acquired cause of the disability. The Finnish disease heritage includes multiple rare diseases, which present as an ID. During the last decades, the average life expectancy of people with an ID has increased (Arvio, Salokivi, & Bjelogrlic-Laakso, 2017), and in contrast to past decades, it is now also possible to evaluate the clinical picture of numerous rare diseases in adult patients.
Muscle-eye-brain disease (MEB) (MIM #253280) is an autosomal recessively inherited disorder characterized by congenital muscular dystrophy, ocular abnormalities, and cortical dysgenesis. MEB belongs to the dystroglycanopathies, a subgroup of diseases called congenital muscular dystrophies (Santavuori et al., 1989; Pihko et al., 1995; Taniguchi et al., 2003). It is caused by homozygous or compound heterozygous pathogenic variants in the POMGNT1 gene. The gene encodes protein-0-mannose-beta-1,2-N-acetylglycosaminiylitransferase and is located on chromosome 1p34.1. All described POMGNT1 mutations lead to complete loss-of-function of enzymatic activity of the POMGNT1 protein, which is responsible for the modification of α-dystroglycan. Mutations in the POMGNT1 gene can cause three different phenotypes: Type A3 (MEB), Type B3 (muscular dystrophy-dystroglycanopathy with mental retardation), and the least severe Type C3 (limb-girdle muscular dystrophy-dystroglycanopathy). Dystroglycan is an essential component of the dystrophin-glycoprotein complex. Dystroglycan has two subunits, α and β. The α-subunit is extracellular and binds to extracellular laminin in the extracellular matrix whereas β-dystroglycan binds to dystrophin inside the cell. A defect in glycosylation of α-dystroglycan causes decreased binding of dystroglycan to laminin and fragility of the cell membrane. This leads to the development of a muscular dystrophy in which skeletal muscle cells are destroyed and serum creatine kinase (CK) is elevated. During brain development, the gene is needed in neurons and oligodendrocytes for neuronal migration (Endo, 2015). Disorders of glycosylation of dystroglycan can cause cortical dysgenesis such as polymicrogyria and ventriculomegaly and visual problems such as congenital myopia and opticus atrophy (Godfrey et al., 2007). In summary, the clinical picture includes severe ID with impaired vision in addition to a slowly progressive muscular dystrophy.
The Finnish founder mutation c.1539 + 1G˃A (p.Leu472_His513del) responsible for MEB was discovered in 2004 (Diesen et al.). This is a splice-site pathogenic mutation in 17 intron, with a substitution of the conserved GT splicing donor sequence to AT. This mutation causes read-through of intronic sequences, resulting in the introduction of a premature stop codon. The mutation also causes skipping of the upstream exon 17, which leads to the deletion of 42 amino acids (Kano et al., 2002). The carrier frequency of the mutation in Finland is estimated at 1/60 and the incidence of the disease at approximately 1/100000 live births (Varilo, 2016).
The clinical manifestation of MEB is a severe disability syndrome with multiple symptoms: intellectual and motor disability, extremely limited communication skills, visual impairment and epilepsy. Babies with MEB are usually born full term. Muscular hypotonia is observed soon after birth. It is most clearly observed in the floppy neck and severe lagging behind of the head when traction is applied to the arms of the baby from a prone position. Motor development is strongly delayed, but some patients learn to walk. By early childhood, spasticity develops in the lower limbs leading to joint contractures. Learning and psychomotor development are slow and the individual has little interest in his/her social environment. Early onset of epilepsy is common, and some patients have clinical signs suggestive of elevated intracranial pressure. Ocular abnormalities vary, but often are profound. Severe congenital myopia is a characteristic symptom. Structural abnormalities in the anterior chamber manifest as a wide spectrum of features including congenital or neonatal glaucoma, retina dystrophy, pallor of the optic discs and clouding of the lenses, which are present in varying combinations. In addition to lack of eye contact, indications of severe visual impairment include involuntary or rapid rhythmic eye movements (nystagmus).
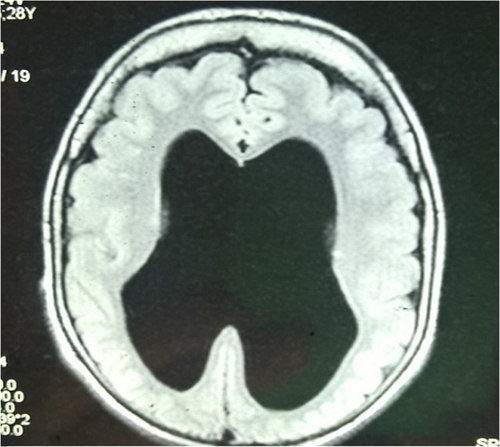
Muscle involvement is indicated by elevated serum CK by the age of 6 months at the latest, but these levels may normalize in adulthood. Electromyography registrations reveal a myopathic result, and muscle biopsy shows muscle dystrophy of varying degree. A low or extinguished electroretinogram is revealed in ophthalmologic examinations, and concurrently flash-VEP (Visually Evoked Potentials) are delayed or enlarged. Electroencephalography (EEG) is often abnormal. Magnetic resonance imaging (MRI) of the head shows cortical malformation characterized by a cobble-stone like cortex, atrophy of the pons as well as enlarged brain ventricles and absent septum pellucidum (Haltia et al., 1997; Haltia, Santavuori, & Somer, 1996; Norio, 2000; Pihko et al., 1995; Santavuori et al., 1989; Santavuori et al., 1998; Vainionpää et al., 2004).
To date, approximately 30 Finnish MEB patients have been identified, half of whom are estimated to be living today.
Clinical reports on MEB mainly describe children and young patients (Borisovna et al., 2019). According to the literature, the average life expectancy of these patients is 10 to 30 years. No long-term follow-up of MEB patients has been published previously. In this article, we describe two unrelated 44-year-old female patients whom the first author (MA) has followed for 38 years, beginning in 1981 when the women were 6-years old. Clinical information and images are published with the consent of the patients' guardians.
2 CLINICAL CASE PRESENTATION
Both women have the homozygous Finnish founder mutation in the POMGNT1 gene. Both are the only MEB patients in their family and they have lived in institutional care since childhood. The MRI scans of the women show cobble-stone malformation of the cortex, a thin corpus callosum, lower vermis hypoplasia, brain stem hypoplasia, absent septum pellucidum and ventriculomegaly (Figure 1). The CK of the first patient at the age of one was 141 U/L and of the second patient at the age of five 548 U/L (upper normal 220 U/L).
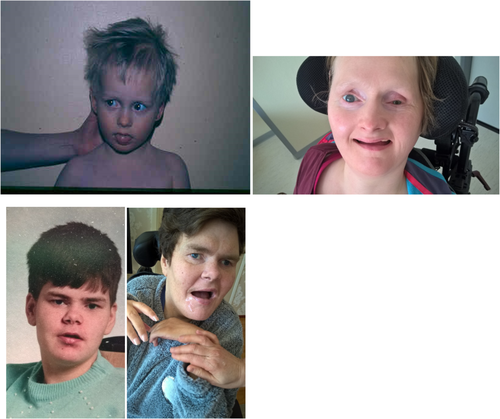
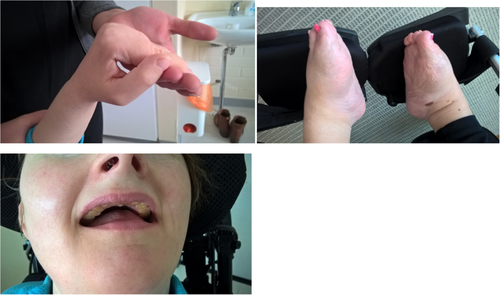
The clinical features of our patients are very similar and have remained quite stable since childhood. Both are profoundly intellectually disabled and unable to communicate, but generally content and peaceful when they are healthy (Figure 2). They enjoy being outdoors, being stroked, and holding vibrating sensory toys. Both have a small head circumference of about 53 cm (-2SD), are about 160 cm tall (-1SD), and their weight fluctuates between 60 and 65 k. In their outward appearance, notice is drawn to lack of eye contact and little reaction to their environment apart from laughter provoked by sudden loud noises. Both women have dilated pupils and a mature cataract in one eye. The first patient has undergone ocular examination under anesthesia in which subluxation of the lenses was observed in the eye without a cataract, initially raising suspicions of a tumor and iris rubeosis. Both women usually have their mouth open revealing excessively worn teeth due to bruxism (Figure 3). Violent movement of the jaw has eroded the teeth of both women. The first patient holds her hands in her lap whereas the second patient almost always cups her chin with her upper limbs, touching her face with her hands (Figure 2). Both women have contractures in the upper limb joints, the first patient in her knuckles (Figure 3) and the second patient in her elbow but they are able to move their limbs and grasp objects. At the age of 20 both women were able to stand holding a frame, but are no longer able to do so. Both women have clubfoot (Figures 3). They are able to roll from lying on their back onto their side, and when assisted to a sitting position they are able to maintain this position for some time. Both, however, have a neck support in their wheelchair or stroller due to sporadic violent head swings (Figure 2). Both women have had repeated bowel obstruction. The first patient eats pureed food with a spoon but requires assistance with drinking; the second patient has been fitted with a gastrostomy tube. Bowel function is ensured with medication.
Both women have antiepileptic monotherapy, levetiracetam, and lamotrigine, respectively. The first patient has not been observed to have an epileptic seizure for years. Her EEG registration of wakefulness shows a high frequency of high-amplitude discharges located in the frontal parts in addition to generalized slowing. The second patient has on average weekly short tonic–clonic seizures accompanied by shouting. Her EEG scans during slow-wave-sleep show a continuous slow spike-sharp-polyspike-slow wave discharge located in the right temporal lobe, which at times becomes more generalized. During times of wakefulness, irritative activity in the right temporal lobe is evident. Interestingly, both women have periods of hypothermia (body temperature 31-34°C) with or without any clear infection or without other causes of hypothermia, such as exposure to cold, hypothyroidism, malnutrition or neuroleptic medication. During these periods of hypothermia, the women mainly sleep. In general, both patients have irregular sleep patterns, and low systolic (76–92 mmHg) and diastolic blood pressure (59–61 mmHg). Hormone treatment has been used to end menstruation.
3 DISCUSSION
Great achievements in the field of genetics have clarified the etiology of rare diseases. Identification of disease-causing mutations provides a molecular basis to the diagnostics of for example, the dystroglycanopathies. This helps clinical practice in many respects, for example, with the prognosis. Molecular genetic analysis showed that two 50-year-old patients with mild ID described in a previous paper (Pihko et al., 1995) were after publication found not to have the presumed POMGNT1 gene mutation and thus they were not suffering from MEB (personal communication, Pihko). The ID caused by the Finnish variant of MEB seems to be severe or profound.
Doctors specialized in ID medicine meet patients with rare diseases almost daily, in contrast to their colleagues in many other fields. Despite this, planning treatment for a patient suffering from a rare disease and estimating the prognosis continues to be challenging primarily for the reason that little published information on adult patients exists and secondly because it is often difficult for intellectually disabled patients to verbally describe their symptoms and sensations. Inability to communicate hampers identification of the causes of evident pain or a behavioral problem. Since the life expectancy of people with an ID has increased during the last decades, it is now possible to complete the clinical picture of many such rare diseases by descriptions of adult patient cases.
Cortical dysgenesias are now better identified due to advances in radiological research methods. The underlying cause of many disabilities previously attributed to acquired pre/perinatal brain damage has in fact been shown to be malformations of the cerebral cortex caused by division, migration, and differentiation of neurons during the fetal period. The underlying cause may be acquired, such as a viral infection or exposure to alcohol during the fetal period or a gene error, an example of which is MEB.
In this article, we describe the clinical features of two middle-aged women suffering from a rare disease. Diesen et al. (2004) briefly described a 37-year-old Finnish male patient with the Finnish POMGNT1 gene mutation, whose mental and motor skills as well as somatic co-morbidities at the age of 28 years appeared similar to our patients.
MEB presents with muscle, eye, and brain symptoms. Our patients' eye abnormalities are characteristic symptoms. In addition to hypotonia, muscular symptoms present as spasticity leading to joint contractures of the limbs. We failed to find any mention of periods of hypothermia in earlier clinical descriptions and suggest this may be a characteristic symptom previously not described. Tendency to hypothermia in our patients presented as tiredness, which is possibly associated with irregular sleep patterns. The thermoregulatory centre located in the hypothalamus may be affected by the severe abnormalities observed in the brain structure and function of MEB patients.
In Finnish textbooks, MEB has been categorized as a neurodegenerative, a progressive, disease of the brain's grey matter (Haltia et al., 1996; Vainionpää et al., 2004). Neuron loss is associated with neurologically progressive diseases. This occurs in lysosomal storage diseases such as aspartylglucosaminuria (AGU), also included in the Finnish disease heritage, in which head circumference may diminish as the skull bone thins and the size of the brain decreases due to atrophy (Arvio, Arvio, Hurmerinta, Pirinen, & Sillanpää, 2005). Previous clinical presentations describe single MEB patients who, due to elevated intracranial pressure, have been fitted with a ventriculo-peritoneal shunt. A patient with neurological symptoms and with abnormally enlarged ventricles may raise suspicions of obstructive hydrocephalia and elevated brain pressure, which could lead to surgical intervention. Cortical dysgenesis is not a progressive state. On the other hand, congenital muscular dystrophy, according to its definition may be a more or less progressive state, in which muscle tissue is replaced with adipose tissue. Unfortunately repeated muscle biopsies were not possible with the two women in this follow-up study, so we do not know in detail to what extent muscular dystrophy has progressed in our patients.
In summary, the clinical features of our patients have been quite stable throughout their lives. There has not been any significant progression of their symptoms. For genetic counseling and the entire community treating patients with MEB this information is valuable. Currently no specific treatment is available. It is important to know the natural history of rare diseases as a point of comparison in future clinical trials of new medicines and other methods of treatment.
ACKNOWLEDGMENTS
We thank the patients, parents, and care workers for participating in this study.
CONFLICT OF INTEREST
Travel grants by PTC Pharma and Biogen to Jaana Lähdetie.




