Loss of CLTRN function produces a neuropsychiatric disorder and a biochemical phenotype that mimics Hartnup disease
Funding information: ACMG Foundation for Genetic and Genomic Medicine; European Regional Development Fund, Grant/Award Numbers: PI15/01082, PI18/0111; National Institutes of Health, Grant/Award Number: T32 GM07526-41
Abstract
Hartnup disease is an autosomal recessive condition characterized by neutral aminoaciduria and behavioral problems. It is caused by a loss of B0AT1, a neutral amino acid transporter in the kidney and intestine. CLTRN encodes the protein collectrin that functions in the transportation and activation of B0AT1 in the renal apical brush bordered epithelium. Collectrin deficient mice have severe aminoaciduria. However, the phenotype associated with collectrin deficiency in humans has not been reported. Here we report two patients, an 11-year-old male who is hemizygous for a small, interstitial deletion on Xp22.2 that encompasses CLTRN and a 22-year-old male with a deletion spanning exons 1 to 3 of CLTRN. Both of them present with neuropsychiatric phenotypes including autistic features, anxiety, depression, compulsions, and motor tics, as well as neutral aminoaciduria leading to a clinical diagnosis of Hartnup disease and treatment with niacin supplementation. Plasma amino acids were normal in both patients. One patient had low 5-hydroxyindoleacetic acid levels, a serotoninergic metabolite. We explored the expression of collectrin in the murine brain and found it to be particularly abundant in the hippocampus, brainstem, and cerebellum. We propose that collectrin deficiency in humans can be associated with aminoaciduria and a clinical picture similar to that seen in Hartnup disease. Further studies are needed to explore the role of collectrin deficiency in the neurological phenotypes.
1 INTRODUCTION
Hartnup disease (OMIM #234500) is an autosomal recessive disorder that affects approximately 1:15,000 live births. It is characterized by intermittent ataxia, chorea, psychiatric disturbances, mild intellectual disability, and a pellagra-like photosensitive rash. However, the majority of affected individuals have a relatively benign course with no clinically significant symptoms except, perhaps, during times of metabolic stress or malnutrition (Bröer, Cavanaugh, & Rasko, 2005).
Biallelic loss-of-function variants in SLC6A19 (OMIM #608893), which encodes the neutral amino acid transporter B0AT1, have been shown to cause Hartnup disease. B0AT1 is expressed in the intestine and kidneys, and loss of B0AT1 function leads to impaired absorption of neutral amino acids which are excreted in the stool and urine (Seow et al., 2004). In addition to aminoaciduria, the loss of these neutral amino acids can lead to deficiencies in precursor metabolites which are responsible for other phenotypes associated with Hartnup disease. The neutral amino acid tryptophan is a precursor for niacin, the deficiency of which causes pellagra (Seow et al., 2004). Tryptophan is also a precursor of serotonin, a monoamine neurotransmitter whose deficiency can result in behavioral and psychiatric phenotypes (Bröer, 2009). These deficiencies, however, are easily overcome in most patients with a regular, protein-rich diet. A study by Malakauskas et al. (2009) also provides evidence that, at least in mice, the body is able to compensate with enhanced protein turnover and fatty acid oxidation.
CLTRN (also known as TMEM27; OMIM #300631) is located on chromosome Xp22.2, and encodes a protein, collectrin, which functions in the trafficking of B0AT1 to the cell surface and its catalytic activation in the kidney and pancreas. In the kidney, B0AT1 requires heterodimerization with collectrin for surface expression and stability (Bröer, 2009). In the intestine, these functions are carried out by angiotensin-converting enzyme 2 (ACE2; OMIM #300335), which is encoded by ACE2 (Danilczyk et al., 2006; Fairweather et al., 2015; Kowalczuk et al., 2008; Malakauskas et al., 2007; Malakauskas et al., 2009; Singer & Camargo, 2011).
CLTRN is highly conserved among species (Akpinar, Kuwajima, Krützfeldt, & Stoffel, 2005; Zhang et al., 2001). In mice, collectrin deficiency causes profound aminoaciduria involving both neutral and charged amino acids (Malakauskas et al., 2007). This expansion in the biochemical phenotype over that seen in Hartnup disease is thought to be the result of collectrin's additional role in the function of the other renal amino acid transporters including, rBAT-b0,+AT, a cationic amino acid transporter, and EAAC1, an anionic amino acid transporter (Malakauskas et al., 2007).
The effects of collectrin deficiency in humans have not been described. Here, we report two patients with aminoaciduria whose phenotypes mimic the biochemical and clinical features seen in Hartnup disease. The first is an 11-year-old male who is hemizygous for a small, interstitial deletion on Xp22.2 that encompasses CLTRN. The second is a 22-year-old male with a deletion spanning exons 1 to 3 of the same gene. Additionally, we provide evidence that CLTRN is expressed in the murine brain.
2 CLINICAL REPORT
2.1 Patient 1
Patient 1 is an 11-year-old male who was seen in the genetics clinic for evaluation of multiple behavioral problems and concerns for Hartnup disease. He was born full-term to nonconsanguineous Caucasian parents and had a birth weight of 3.54 kg (65th centile). At 2 years of life, he started having absence seizure-like episodes. However, his EEG was negative. An incidental urine amino acid analysis revealed elevated threonine, serine, asparagine, glutamine, proline, glycine, alanine, tyrosine, phenylalanine, tryptophan, serine, and histidine (Table 1). A plasma amino acid analysis obtained at the same time was normal (Table 2). Due to the significant neutral aminoaciduria, he was given a provisional diagnosis of Hartnup disease and was placed on niacin supplementation. Since he was clinically asymptomatic, parents stopped niacin supplementation at 3 years of age.
| Amino acid | Polarity/charge | Abnormality expected in Hartnup disease | Patient 1 | Patient 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2 years (reference range: μmol/g creatine) | 7 years (reference range: mmol/mol creatine) | 11 years (reference range: μmol/g creatine) | 9 years | 16 years | 22 years | Reference range μmol/g creatine | |||
| O-Phosphoserine | Normal | 130 (0–379) | 185 | 62 | 0–152 | ||||
| O-Phosphoethanolamine | Normal | 54 (0–841) | 102 | 0 | 41 | 0–83 | |||
| Taurine | Normal | 217 (140–1,520) | 65 (≤255) | 96 (37–3,467) | 889 | ||||
| Aspartic acid | Polar/anionic | Normal | 30 (0–120) | 1 (≤2) | 5 (0–217) | 38 | 0 | 7 | 0–128 |
| Threonine | Polar/neutral | Elevated | 1,059 (30–290) | 68 (4–60) | 1,781 (25–7,040) | 3,520 | 1,841 | 1,910 | 70–370 |
| Serine | Polar/neutral | Elevated | 3,381 (200–760) | 202 (13–127) | 2,572 (91–1,339) | 6,630 | 2,351 | 2,986 | 158–1,010 |
| Asparagine | Polar/neutral | Elevated | 2,157 (72–332) | 63 (3–42) | 1,495 (11–514) | 3,197 | |||
| Glutamic acid | Polar/anionic | Normal | 50 (0–120) | 5 (≤10) | 29 (0–101) | 12 | 0 | 31 | 0–100 |
| Glutamine | Polar/neutral | Elevated | 6,029 (240–880) | 422 (18–388) | 3,649 (51–2,153) | 8,070 | 2,532 | 2,410 | 110–1,380 |
| 2-Aminoadipic acid | Normal | 9 (≤34) | 0 (0–195) | 165 | |||||
| Proline | Nonpolar/neutral | Elevated | 211 (30–110) | 5 (≤11) | 67 (0–335) | 434 | 0 | 23 | 0–120 |
| Glycine | Nonpolar/neutral | Elevated | 2,679 (500–2,450) | 157 (23–413) | 2,405 (264–6,017) | 2,925 | 1,149 | 2,955 | 700–2,100 |
| Alanine | Nonpolar/neutral | Elevated | 2,493 (90–830) | 80 (8–156) | 1,121 (53–1,427) | 1,588 | 1,066 | 1,448 | 120–1,280 |
| Citrulline | Normal | 58 (10–30) | 1 (≤4) | 58 (0–93) | 135 | ||||
| 2-amino n-butyric | Normal | 28 (5–136) | 59 | ||||||
| Valine | Nonpolar/neutral | Elevated | 66 (10–110) | 8 (2–20) | 65 (13–183) | 146 | 56 | 58 | 20–110 |
| Cystine | Normal | 55 (20–80) | 27 (0–160) | 107 | 62 | 77 | 15–150 | ||
| Methionine | Nonpolar/neutral | Elevated | 47 (10–130) | 1 (≤5) | 10 (0–196) | 132 | 50 | 15 | 0–80 |
| Isoleucine | Nonpolar/neutral | Elevated | 21 (10–90) | 2 (≤5) | 7 (4–163) | 10 | 17 | 19 | 0–130 |
| Leucine | Nonpolar/neutral | Elevated | 63 (20–140) | 4 (≤13) | 8 (4–176) | 39 | 49 | 49 | 0–90 |
| Tyrosine | Nonpolar/neutral | Elevated | 622 (60–300) | 35 (3–48) | 571 (9–403) | 1,312 | 437 | 334 | 100–600 |
| Phenylalanine | Nonpolar/neutral | Elevated | 214 (30–150) | 10 (2–22) | 65 (8–227) | 251 | 113 | 84 | 25–150 |
| β-Alanine | Normal | 1(≤5) | 31 (0–422) | ||||||
| Tryptophan | Nonpolar/neutral | Elevated | 380 (0–140) | 18 (2–27) | |||||
| β-Aminoisobutyric | Normal | 8 (≤133) | 47 (0–1,985) | 137 | |||||
| Ornithine | Normal | 12 (0–110) | 1 (≤5) | 27 (0–170) | 33 | 12 | 13 | 0–88 | |
| Lysine | Basic polar/cationic | Normal | 351 (200–400) | 19 (3–112) | 370 (8–557) | 637 | 293 | 258 | 27–796 |
| 1-Methylhistidine | Normal | 145 (0–350) | 15 (11–40) | 462 (0–2,550) | 38 | ||||
| Histidine | Basic polar/cationic | Normal | 6,629 (440–1,520) | 206 (9–426) | 4,111 (58–3,022) | 3,745 | 1,404 | 2,674 | 80–1,500 |
| 3-Methylhistidine | Normal | 202 (190–450) | 250 (19–1,124) | 102 | |||||
| Arginine | Basic polar/cationic | Normal | 30 (20–100) | 3 (≤8) | 27 (0–117) | 66 | 23 | 24 | 0–71 |
| Camosine | Normal | 423 (72–402) | |||||||
| Homocystine free | Normal | 1 (<1) | 0 (0–1) | ||||||
- Values above the reference range are shown in bold.
| Plasma amino acids | Patient 1 (reference range in μmol/L) | Patient 2 | ||
|---|---|---|---|---|
| Preprandial | Postprandial | Reference range in μmol/L | ||
| O-Phosphoserine | 5 (0–15) | |||
| O-Phosphoethanolamin | 2 (0–32) | |||
| Taurine | 86 (5–127) | 46 | 48 | 30–150 |
| Aspartic acid | 4 (1–21) | 3 | 4 | 2–20 |
| Threonine | 151 (34–161) | 141 | 203 | 78–197 |
| Serine | 105 (57–169) | 136 | 159 | 92–97 |
| Asparagine | 59 (7–82) | 62 | 89 | 31–120 |
| Glutamic acid | 39 (3–89) | 40 | 36 | 5–80 |
| Glutamine | 573 (266–746) | 523 | 641 | 330–632 |
| Proline | 359 (39–332) | 195 | 417 | 90–270 |
| Glycine | 218 (92–346) | 276 | 320 | 109–293 |
| Alanine | 439 (103–528) | 285 | 495 | 167–439 |
| Citrulline | 19 (6–38) | 26 | 22 | 8–34 |
| 2-Amino n-butyric acid | 14 (3–34) | 26 | 28 | 5–31 |
| Valine | 263 (82–293) | 231 | 316 | 102–294 |
| Cystine | 14 (7–43) | 38 | 18 | 15–59 |
| Methionine | 31 (7–34) | 29 | 46 | 12–37 |
| Isoleucine | 88 (16–89) | 77 | 110 | 32–90 |
| Leucine | 152 (35–164) | 140 | 210 | 57–155 |
| Tyrosine | 96 (19–100) | 71 | 111 | 39–87 |
| Phenylalanine | 64 (25–82) | 57 | 85 | 40–70 |
| Homocystine free | 0 (0–1) | |||
| Ornithine | 75 (5–100) | 55 | 116 | 30–109 |
| Lysine | 176 (41–225) | 159 | 260 | 112–240 |
| 1-Methylhistidine | 6 (0–14) | |||
| Histidine | 84 (38–103) | 46 | 71 | 45–104 |
| 3-Methylhistidine | 2 (0–12) | |||
| Arginine | 82 (18–127) | 78 | 95 | 47–122 |
| Alloisoleucine | 0 (0–1) | |||
| Argininosuccinic | 0 (0–1) | |||
| Tryptophan | 64 | 95 | 47–122 | |
At around 4 years of age, he started having multiple behavioral problems including anxiety, odd fears, and compulsions. He also had brief episodes of motor tics including shoulder-shrugging, neck-extension, jaw jutting, sniffing, facial grimacing, and oculogyric movements. Over time, he developed difficulty with writing and was noted to have poor coordination for which he received occupational and physical therapies. He was evaluated by a neurologist at 6 years of age for the behavioral concerns and involuntary movements and was diagnosed with obsessive–compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD). He was started on medications including dextroamphetamine/amphetamine, methylphenidate, sertraline, fluoxetine, and atomoxetine without any clinical improvement. Subsequent IQ testing revealed a full-scale IQ (FSIQ) of 126. He had a normal brain MRI. Repeat urine amino acid analysis showed mild elevation in serine, threonine, asparagine, and glutamine (Table 1). Since he did not have significant neutral aminoaciduria, the diagnosis of Hartnup disease was removed.
He was referred to a clinical geneticist at 11 years of age for evaluation of his behavioral abnormalities and aminoaciduria. Mother reported an improvement in involuntary movements and behavioral abnormalities which were only evident during periods of stress. His growth parameters at the age of 11 years included a weight of 44 kg (87th centile), height of 152.4 cm (92nd centile), and head circumference of 56.8 cm (98th centile). His physical examination did not reveal any dysmorphic features except for macrocephaly, a slightly hypoplastic left helix, and a few facial lentigines. His repeat urine amino acids showed abnormal values similar to those seen in prior testing with elevations in neutral amino acids including serine, asparagine, glutamine tyrosine, and histidine (Table 1). Plasma amino acids, urine organic acids, acylcarnitines, and fragile X testing were unremarkable.
2.2 Patient 2
Patient 2 is a 22-year-old male who was seen for the first time at 8 years of age in a neuropediatric clinic for evaluation of behavioral and learning problems. He was born full-term with a birth weight of 3.95 kg (95th centile). Since he was 18 months old, he had frequent tantrums, poor social contact, and repetitive behaviors. During the transition from preschool to school, he felt awkward in social situations, showed poor use of body language and a lack of comprehension of emotions and jokes. He was subsequently diagnosed with Asperger syndrome at 9 years of age. At that time, his physical and neurological examinations were normal as were his growth parameters: weight: 36.5 kg (75th centile); height: 138.5 cm (75th centile); head circumference: 53 cm (75th centile). His total IQ was 75 (verbal 82, perceptual reasoning 83, working memory 75, and processing speed 79) with prominent difficulties in executive functions, visuoconstructional, and pragmatic language skills. A brain MRI was unremarkable.
Because of his borderline intellectual disability, a metabolic workup was performed including a urine amino acid analysis that was remarkable for elevations of threonine, serine, asparagine, glutamine, glycine, alanine, tyrosine, and histidine (Table 1). Plasma amino acid levels were normal (Table 2). Additionally, fragile X testing was negative. A detailed clinical anamnesis revealed a photosensitive skin rash (Figure 1a) and ocular erythema as well as frequent diarrhea, particularly in times of stress. A cerebrospinal fluid test revealed normal levels of amino acids (tryptophan was not included) and low levels of 5-HIAA (69 nmol/L; normal 87–366), the final metabolite product of serotonin. He was given a diagnosis of Hartnup disease and was placed on niacin supplementation (100 mg/day), a high protein diet (2 g/kg/day) and 5-hydroxytryptophan (gradual increase from 1 to 5 mg/kg/day). His attention span, cognitive flexibility, social interaction, and skin photosensitivity improved during the first year of treatment. Later on, he experienced depression and anxiety-like episodes, so 5-hydroxytryptophan was replaced by fluoxetine with remarkable improvement. He also experienced social integration problems, anxiety crises, and bouts of soliloquy. He was followed carefully for psychological and psychiatric symptoms and received additional treatment with quetiapine and valproate as mood stabilizers. When he forgets to take his niacin supplement, the photo-induced skin lesions reappear. His urine amino acid pattern has remained unchanged over time (Table 1). Preprandial and postprandial plasma amino acids remained normal (Table 2).
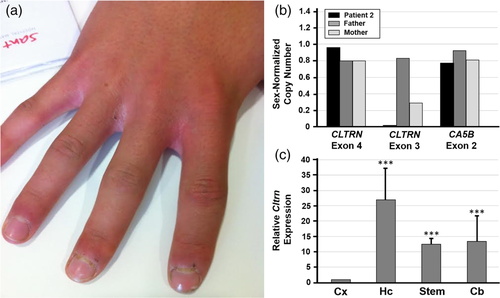
At his most recent evaluation, at the age of 22 years, he was autonomous with regards all aspects of his life: he worked as a gardener, lived alone, had a group of friends, and enjoyed a very good relationship with his family. His anthropometric measurements were within normal limits: weight 73 kg (58th centile), height: 172.5 cm (27th centile).
3 RESULTS AND METHODS
3.1 Molecular evaluation
An array-based copy number variant (CNV) analysis on Patient 1 revealed a deletion of approximately 0.044 Mb on chromosome Xp22.2 (minimum interval chrX:15,676,526–15,720,182; maximum interval chrX:15,676,482–15,720,880; hg19). This region contains two RefSeq genes; CLTRN (OMIM #300631) and the pseudogene CA5BP1 (Figure 2). A 0.17 Mb copy number gain on chromosome 6q26 (minimum interval chr6:162,757,035–162,926,694; maximum interval chr6:162,687,507–162,972,023; hg19) was also identified. This interval contains the second exon of PARK2 (OMIM #602544) which encodes the E3 ubiquitin ligase protein parkin. The inheritance patterns of these CNVs remain undetermined and the patient has since been lost to follow-up.
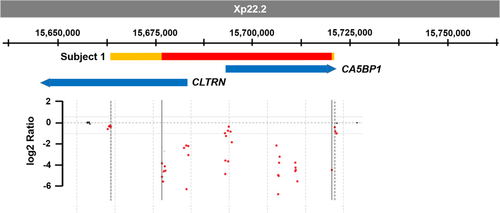
The array-based CNV analysis of whole-exome sequencing (WES) data from Patient 2 revealed a deletion in CLTRN gene (chrX:15,677,140–15,682,898; size 5,758 bp; hg19), that includes exons 1 to 3 of the gene (NM_020665.6). These exons encode the first 106 amino acids of CLTRN which includes the extracellular domain and the signal peptide. If a protein were expressed from this allele, we would predict it to be a truncated, nonfunctional protein that is improperly localized.
The validation of this deletion was achieved by quantitative PCR. Specifically, primers designed to amplify a region in exon 3 of CLTRN failed to amplify from cDNA generated from Patient 2 (Figure 1b). This is consistent with a hemizygous deletion of this region. In contrast, amplification of a region in CLTRN exon 4 and a region in exon 2 of the downstream CAS5B gene (NM_007220.4) revealed a normal male copy number. These tests also revealed that his father has a normal male copy number in this region and that his mother is a heterozygous carrier of the deletion (Figure 1b). The 5′ and 3′ breakpoints of the deletion were not established.
3.2 Analysis of Cltrn expression in mice brain
To determine if Cltrn is expressed in the murine brain, we performed quantitative RT-PCR on samples obtained from the whole brain, cortex, hippocampus, brain stem, and cerebellum of C57BL/6 wild-type mice. Total brain Cltrn expression was found to be 10,000 times lower than kidney expression (data not shown), but still detectable in reliable CT (cycle threshold) values and consistent within replicates and animals. Among specific brain areas, the expression of Cltrn was significantly higher in the hippocampus, brain stem, and cerebellum than in the cortex (Figure 1c).
3.3 Methods
3.3.1 Biochemical and molecular studies
Plasma and urinary amino acids were determined in CLIA/CAP certified clinical reference laboratories. A total of 100 μl of plasma or appropriate urine amount were precipitated with Seraprep or Uriprep (Pickering Laboratories, Mountain View, CA). Supernatants were filtered and concentrations were measured by amino acid analyzers. Urinary amino acids were measured by ion-exchange chromatography with ninhydrin derivatization in a Biochrom 30 analyzer (Chromsystems, Gräfelfing, Germany). Urinary excretion of amino acids was normalized to the creatinine content and results were compared with the reference intervals established in both hospitals.
Array-based CNV analysis was performed according to the manufacturer's instructions on DNA extracted from whole blood using a Baylor Genetics version 11.2 custom 400 K Agilent oligonucleotide array (Agilent Technologies, Santa Clara, CA; Pillai et al., 2018).
Whole exome sequencing (WES) was performed in CNAG-CRG with Nimblegen SeqCap EZ MedExome 47 Mb and sequenced on an Illumina HiSeq 2000 with 2x100 base pairs paired-end. Bioinformatic processing of the sample was done according to CNAG-CRG home-made pipeline with the reference genome hg19/GRCh37. The tools used for single nucleotide variants (SNV) and small insertions or deletions (indel) analysis were BWA-MEM v.0.7.15, Picard MarkDuplicates v1.118, GATK v3.6, GATK HaplotypeCaller v3.6, and SnpEff v4.3. The tools used for CNV analysis were ExomeDepth, Conifer, and XHMM.
For the validation of the CNV in Patient 2, oligonucleotides were designed to amplify three regions in and around the deletion (CLTRN exon 4, CLTRN exon 3, and CA5B exon 2). qPCR was performed on DNA samples from Patient 2 and his parents using GoTaq qPCR MasterMix (Promega) on an Applied Biosystems 7500 Real-Time PCR System. Specific oligonucleotide sequences corresponding to CLTRN exon 4 are, in 5′-3′ sense: (F) TGCAGAATTTCCCATGTCCT and (R) CCTTATGGCTGATTGCACCT, for CLTRN exon 3: (F) TGCCTGGGATACCAATGAA and (R) TTGCTTCTCTGTTGGGAACT, and for CA5B: (F) GCCTGAGGGTCATTCTTCAA and (R) CGGTTCCGGGTTTTGTAAGT. Values were normalized using sex-matched controls.
3.3.2 RNA extraction and qRT-PCR analysis
RNA for qRT-PCR was obtained from the brains of three different C57BL/6 wild-type mice (n = 3) using standardized methods. Specifically, brain specimens were conserved in RNALater (Thermofisher, Waltham, MA) at −20°C for 24 hr. RNA was then extracted using an RNeasy® Fibrous Tissue Mini Kit (Qiagen), following the manufacturer's instructions. Total RNA was eluted in 30 μl of RNAse-free water and stored at −80°C. RNA concentration was measured using the NanoDrop 2000 Spectrophotometer (ThermoScientific).
RT-qPCR studies were carried out following a two-step protocol. First, cDNA was synthesized from a total of 500 ng of RNA per reaction following the recommendations provided with SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen™).
Second, qPCR was performed in a QuantStudioTM 6 Flex Real-Time PCR System (Applied Biosystems™) with PowerUpTM SYBRTM Green Master Mix (Applied Biosystems™). Data were analyzed using a comparative method, correlating the initial template concentration with the cycle threshold (Ct) to obtain the relative quantity (RQ) of RNA. The RQ is defined as 2−ΔΔCt, where ΔΔCt is the ΔCt of the specimen cell line minus the ΔCt of the control cell line, and ΔCt is the Ct of the target gene minus Ct of the endogenous gene (RPLP0). GABRA1 expression was assessed as a positive control for brain versus kidney gene expression (data not shown).
The probe's design was performed using DNA sequence corresponding to exonic regions present in Cltrn transcripts. Primers were, in 5′–3′ sense: (F) CTGTGCCCGTCTGGATTATT and (R): GTTGCCGGATTCCAGATAGA.
4 DISCUSSION
Here, we present two patients, an 11-year-old male with a small, hemizygous deletion encompassing CLTRN that encodes collectrin, and a 22-year-old male with a deletion spanning exons 1–3 of the same gene. Since Patient 1 was lost to follow-up after the initial clinic visit, the inheritance of the deletion could not be identified. In Patient 2, the deletion was maternally inherited.
Since B0AT1 is dependent on collectrin for transportation to the cell membrane and activation in renal epithelial cells (Danilczyk et al., 2006), it comes as no surprise that the two patients would have loss of neutral amino acids in the urine due to impaired absorption in the proximal tubules. Unlike SLC6A19, whose deficiency causes Hartnup disease, collectrin is not expressed to the same degree in the small intestinal brush border epithelium. Rather, ACE2 is associated with surface expression and activation of B0AT1 in the intestine (Camargo et al., 2009). Therefore, in CLTRN deficiency, intestinal uptake of neutral amino acids is expected to be normal.
The aminoaciduria classically observed in Hartnup disease includes loss of the neutral amino acids such as tryptophan, alanine, asparagine, glutamine, histidine, isoleucine, leucine, phenylalanine, serine, threonine, tyrosine, and valine. However, the branched-chain amino acids such as valine, leucine, and isoleucine were consistently within the normal range on all three urine amino acid measures in both patients. It has been reported that B0AT2, a sodium-dependent neutral amino acid transporter with properties similar to B0AT1, favors the transport of branched-chain amino acids and proline compared to other neutral amino acids (Bröer et al., 2006; Takanaga, Mackenzie, Peng, & Hediger, 2005). Even though early studies detected the presence of B0AT2 only in the brain, subsequent studies supported its presence in a different part of the proximal collecting tubule of the kidney and its role in branched-chain amino acid absorption (Bröer et al., 2006). The role of collectrin in the expression and stabilization of B0AT2 has not been described. We hypothesize that the functionally intact B0AT2 in the collecting duct along with B0AT1–ACE2 complex in the intestine could explain the normal branched-chain amino acid levels in both plasma and urine in our patients. Similarly, histidine transport is pH-dependent. This could be further explained by the role of collectrin in the function of other amino acid transporters of the kidney, including rBAT-b0,+AT and EAAC1, which serve as cationic and anionic amino acid transporters respectively. Specifically, Malakauskas et al. (2007) reported that hemizygous Cltrn knockout male mice not only had significantly reduced expression of B0AT1, but also reduced expression of rBAT-b0,+AT. They also reported increased intracellular sequestration of EAAC1 protein even though no significant difference was appreciated at the plasma membrane (Malakauskas et al., 2007).
Plasma amino acids were persistently within the normal range for both patients, even during times of stress and when neurological symptoms were worsening. Postprandial plasma amino acid quantification in Patient 2 showed a nonspecific generalized amino acid elevation as expected (Table 2). The nonspecific elevations include neutral amino acids such as phenylalanine, tyrosine, alanine, proline, glutamine, threonine, and serine and the branched-chain amino acids including leucine, isoleucine, and valine. This evidence supports the facts that intestinal uptake of neutral amino acids is not impaired in collectrin deficiency. This could in part be due to the suspected normal B0AT1 function in the intestine in addition to other natural compensatory mechanisms observed in Hartnup disease. Renal amino acid reabsorption is defective in these individuals and is the likely cause of aminoaciduria. However, a photosensitive dermatitis appeared in Patient 2, in particular, when niacin was discontinued. Further studies regarding CLTRN and B0AT1 functions in the skin will be required to explain this phenomenon.
A search of >80,000 samples referred for exome sequencing or array-based CNV analyses at Baylor Genetics did not reveal additional individuals with hemizygous or homozygous CLTRN loss-of-function variants or deletions. Two individuals carrying a deletion involving CLTRN were identified in the Database of Genomic Variants (DGV; http://dgv.tcag.ca/dgv/app/home; accession numbers: esv2758862, essv4930) (MacDonald, Ziman, Yuen, Feuk, & Scherer, 2014; Redon et al., 2006). In addition, three deletions (two intronic and one involving a coding exon) in CLTRN and nine hemizygous loss-of-function variants were reported in gnomAD (https://gnomad.broadinstitute.org/; Karczewski et al., 2019). Two males with larger deletions (55–152 Mb) involving CLTRN were reported in the DECIPHER database (https://decipher.sanger.ac.uk/; Patient ID: 284367 and 276716; Firth et al., 2009). However, aminoaciduria was not mentioned in their phenotypic descriptions. The loss of function variants and deletions in DGV and gnomAD could be explained by milder phenotypes associated with CLTRN deletion that may go unrecognized.
The neuropsychiatric phenotype in our patients deserves special attention. Normal levels of plasma amino acids, and the normal CSF amino acid profile in Patient 2, point toward an alternative mechanism responsible for the neurological signs. Low levels of cerebral 5-HIAA in the Patient 2, and the positive response to treatments that increase serotonin concentration in the brain, suggest that CLTRN could play a role in regulating neurotransmission. We could detect Cltrn transcripts in brain (Figure 1c) and this is in line with the already described expression of ACE2 in the nervous system (Komatsu et al., 2002). ACE2 is a protein consisting of two domains; the N-terminus is a carboxypeptidase homologous to angiotensin-converting enzyme (ACE) and the C-terminus is homologous to collectrin (Alenina & Bader, 2019). Additionally, CLTRN has been found in secretory vesicles and in proximity to vesicle/membrane fusion events in pancreatic β cells (Akpinar et al., 2005), and it binds to soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE), a protein complex involved in intracellular movement of vesicles and membrane proteins. Specifically, in mIMCD3 cells, CLTRN co-precipitates with the SNARE proteins snapin, SNAP-23, and syntaxin-4 (Zhang et al., 2007). The SNARE complex plays a crucial role in synaptic vesicle trafficking and neurotransmitter release. Further studies connecting CLTRN and SNARE proteins in neurons are needed to better understand the involvement of CLTRN in neurotransmission. Interestingly our study shows that Cltrn is expressed in the murine brainstem and cerebellum which have an extensive serotoninergic innervation.
Since loss-of-function variants in PARK2 cause autosomal recessive Type 2 Juvenile Parkinson disease (OMIM #600116), and CNVs affecting PARK2 have been described in individuals with neurodevelopmental disorders (Mariani et al., 2013; Palumbo et al., 2016), we looked into the possibility that the PARK2 exon 2 duplication could be contributing to the behavioral problems in Patient 1. However, a recent study by Conceição et al. suggests that primarily CNV's targeting the C-terminal exons, corresponding to the ring-between-ring (RBR) domain, are responsible for the neurodevelopmental symptomology, as opposed to N-terminal CNV's that are just as common in healthy controls (Conceição et al., 2017). Since the CNV of Patient 1 only involved exon 2 in the N-terminal region of the gene, it seems less likely to be the sole cause of his behavioral problems. Although additional studies, including parental CNV analyses and exome sequencing, were suggested as a means of identifying the inheritance patterns of the proband's CNVs and the underlying cause of his behavioral problems, these tests were not obtained and the Patient 1 was subsequently lost to follow-up.
In summary, we conclude that collectrin deficiency in humans causes a particular pattern of aminoaciduria, psychiatric/behavioral phenotypes, and an intermittent photosensitive dermatitis similar to that seen in Hartnup disease. However, the underlying pathophysiological mechanism remains unclear since plasma and CSF amino acid levels remain within the normal range. The identification and evaluation of additional individuals with loss of collectrin function may help to clarify both the biochemical phenotype and clinical consequences associated with this disorder.
ACKNOWLEDGEMENTS
This work was funded in part by a Sanofi Genzyme ACMGF Next Generation Training Award to NRP and a National Institutes of Health grant (T32 GM07526-41) to BJS, and by the Departament de Salut de la Generalitat de Catalunya (PERIS SLT002/16/00174) to FP. AGC is funded by FIS: PI15/01082 and PI18/0111 (Instituto de Salud Carlos III: ISCIII and Fondo Europeo de desarrollo regional, FEDER).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
EDITORIAL POLICIES AND ETHICAL CONSIDERATIONS
This study was conducted in accordance with the ethical standards of the institution's committee on human research and was in keeping with international standards.
Open Research
DATA AVAILABILITY STATEMENT
Clinical and molecular data included in this manuscript have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/).




