An unusual cause for Coffin–Lowry syndrome: Three brothers with a novel microduplication in RPS6KA3
Abstract
Coffin–Lowry syndrome (CLS) is a rare X-linked disorder characterized by moderate to severe intellectual disability, hypotonia, craniofacial features, tapering digits, short stature, and skeletal deformities. Using whole exome sequencing and high-resolution targeted comparative genomic hybridization array analysis, we identified a novel microduplication encompassing exons five through nine of RPS6KA3 in three full brothers. Each brother presented with intellectual disability and clinical and radiographic features consistent with CLS. qRT-PCR analyses performed on mRNA from the peripheral blood of the three siblings revealed a marked reduction of RPS6KA3 levels suggesting a loss-of-function mechanism. PCR analysis of the patients’ cDNA detected a band greater than expected for an exon 4–10 amplicon, suggesting this was likely a direct duplication that lies between exons 4 through 10, which was later confirmed by Sanger sequencing. This microduplication is only the third intragenic duplication of RPS6KA3, and the second and smallest reported to date thought to cause CLS. Our study further supports the clinical utility of methods such as next-generation sequencing and high-resolution genomic arrays to detect small intragenic duplications. These methods, coupled with expression studies and cDNA structural analysis have the capacity to confirm the diagnosis of CLS in these rare cases.
1 INTRODUCTION
In 1966, the first identified patient to have Coffin–Lowry syndrome (CLS) was described by Coffin, Siris, and Wegienka 1966, and then independently described again by Lowry, Miller, and Fraser (1971). The condition is a rare, X-linked inherited disorder that is characterized by dysmorphic features and intellectual disability in males, with mild expression in some females (Pereira, Schneider, Pannetier, Heron, & Hanauer, 2010). Characteristic facial features include prominent forehead and ears, downward slanting palpebral fissures, ocular hypertelorism, broad-based nose with anteverted nares, thick nasal septum, wide mouth, full lips, and dental anomalies (Delaunoy et al., 2001; Pereira et al., 2010; Rogers & Abidi, 1993; Rojnueangnit, Jones, Basehore, & Robin, 2014). These facial features are typically mild during infancy and become more pronounced in late childhood and adolescence (Delaunoy et al., 2001). Other features include behavioral problems, growth restrictions, and various skeletal anomalies including progressive childhood-onset kyphoscoliosis, pectus anomalies, narrow pelvis, tapered fingers, and metacarpal pseudoepiphyses (Pereira et al., 2010; Rogers & Abidi, 1993). In addition, there can be neuropsychiatric concerns including progressive spasticity, sleep apnea, loss of strength, and stimulus-induced drop attacks, which can be triggered by multiple events including unexpected tactile or auditory stimuli, or excitement (Rogers & Abidi, 1993).
Pathogenic mutations in the gene RPS6KA3 (MIM:300075), located at Xp22.12, cause CLS (MIM:303600; Pereira et al., 2010). RPS6KA3 is composed of 22 exons and encodes a protein of 740 amino acids (Delaunoy et al., 2001; Jacquot et al., 1998; Pereira et al., 2010). The protein, in turn, functions as a growth-factor regulated protein kinase, ribosomal S6 kinase 2 (RSK2). Related to RSK2 are three other proteins, RSK1, RSK3, and RSK4; all of which are from four different genes (Delaunoy et al., 2001). This family of genes is part of the Ras-MAPK signaling pathway (Delaunoy et al., 2001; Pereira et al., 2010; Wang et al., 2006). There is a broad range of cellular signals that activate these proteins including insulin, growth factors, neurotransmitters, and UV-irradiation. Once activated, the proteins are involved in cellular proliferation, differentiation, and apoptosis (Delaunoy et al., 2001; Wang et al., 2006). RPS6KA3 expression is seen at its highest level in regions of the brain that have high synaptic activity (Rojnueangnit et al., 2014).
The types of mutations in RPS6KA3 vary and either lead to loss of phosphotransferase activity of the protein or a premature termination of translation (Delaunoy, Dubos, Marques Pereira, & Hanauer, 2006). The most commonly reported pathogenic variants include missense mutations, nonsense mutations, splicing errors, or small deletion or insertion events leading to frameshift. There have been a few reported intragenic deletions and a few multiple gene duplications that include RPS6KA3 (Pereira et al., 2010). No genotype–phenotype correlations have been established for CLS (Wang et al., 2006).
We describe three brothers with the clinical and radiographic findings of CLS, each of whom possess a duplication of approximately 7 kb encompassing exons 5 through 9 in RPS6KA3. This is the third intragenic duplication to be described in RPS6KA3 and the second as causative for CLS. The duplication reaffirms that intragenic rearrangement, not detectable by common exon screening, can cause CLS. Therefore, in cases where suspected CLS is not confirmed by traditional methods, additional testing for intragenic duplications in RPS6KA3 should be considered.
2 CLINICAL REPORT
Individuals III-1, III-2, and III-3 (Figure 1a) are brothers with similar clinical and radiographic features that are typical for CLS, and each possess the same duplication of exons 5 through 9 in RPS6KA3. They are the only children of non-consanguineous Caucasian parents. The patients’ mother, II-4, has a history of developmental delay and mild intellectual disability that required special education. She also has unilateral deafness and mental health issues, including suspected bipolar disorder and depression. It is believed that she is a carrier of this intragenic duplication but she was not available to confirm this prediction. The patients’ father, II-3, has an early-stage macular degeneration but no intellectual disability. A maternal half-uncle, II-8, has autism spectrum disorder, attention-deficit/hyperactivity disorder, learning disabilities, and complete deafness. However, he is not thought to have CLS, and the etiology of his problems is unknown. There also are three other maternal uncles, II-5, II-6, II-7, with no intellectual disability or learning difficulties.
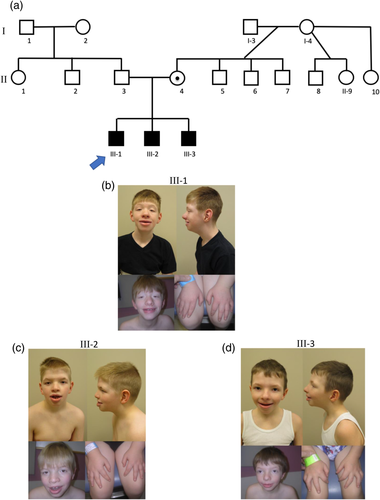
2.1 Individual III-1
Individual III-1 (Figure 1b) is a 12-year-old male, born full term, after an uncomplicated pregnancy. The biological mother smoked tobacco during the pregnancy but reportedly did not use other substances. At birth he had a submucous cleft palate, laryngomalacia, and vesicoureteral reflux and subsequently experienced aspiration. Shortly after birth, in 2006, a chromosome analysis at band level G636 determined his karyotype to be 46,XY. At age 4 years, he started having febrile seizure during sleep, which did not reoccur after being treated with topiramate (Topamax). At 5, he was first evaluated by clinical genetics because of global developmental delay, marked speech and language delay, short stature, microcephaly, and other dysmorphic features. At that time, in 2011, a comparative genomic hybridization (CGH) array using the SignatureChipOS v2.0 3-plex array which included 134,811 oligonucleotide probes, was normal with no significant copy number changes detected. Prader–Willi syndrome methylation studies along with 22q11.2 deletion syndrome FISH, Prader–Willi syndrome FISH, and subtelomeric FISH studies were normal. On physical examination, he had short palpebral fissures, ptosis, broad-based nose, hypodontia, bifid uvula, supernumerary nipples, pectus carinatum, and broad-tapered fingers (Figure 1b and Table 1). A hearing evaluation identified a possible conductive hearing loss. A spinal radiograph indicated moderate neuromuscular scoliosis, which has required bracing. A spinal MRI revealed thoracolumbar spinal cord syrinx extending from T6 to T12, two small annular fissures at C5–C6 and C6–C7, and a small central disc protrusion at C6–C7. There was no significant spinal canal stenoses or other cord abnormalities. A sleep study revealed mild obstructive sleep apnea. At age 11 years, his height was 132.8 cm (−2.2 SD). At age 12 years, his height was 149.0 cm (19 percentile), weight was 38.40 kg (18 percentile), and head circumference was 51.0 cm (3 percentile). He still does not have any speech. Cognitively, he is more severely affected than his brothers.
| Subjects | |||||
|---|---|---|---|---|---|
| III-1 | III-2 | III-3 | Total | Observed in CLS (%)a | |
| Moderate intellectual disability | + | + | + | 3/3 | 80–99 |
| Global developmental delay | + | + | + | 3/3 | 80–99 |
| Speech delay | + | + | + | 3/3 | 80–99 |
| Behavioral problems/neuropsychiatric concerns | − | − | − | 0/3 | 5–29 |
| Febrile seizures | + | − | − | 1/3 | 5–29 |
| Stimulus-induced drop attacks | − | − | + | 1/3 | 20 (Rogers & Abidi, 1993) |
| Prominent forehead | − | − | − | 0/3 | 80–99 |
| Epicanthus | − | − | − | 0/3 | 80–99 |
| Downward slanting palpebral fissures | − | + | − | 1/3 | 80–99 |
| Short palpebral fissures | + | − | + | 2/3 | − |
| Ptosis | + | − | + | 2/3 | − |
| Ocular hypertelorism | + | − | − | 1/3 | 80–99 |
| Pseudo-esotropia | − | + | − | 1/3 | − |
| Depressed nasal bridge | − | − | − | 0/3 | 80–99 |
| Broad-based nose | + | + | + | 3/3 | 30–79 |
| Anteverted nares | − | − | − | 0/3 | 80–99 |
| Dental abnormalities | + | + | + | 3/3 | 80–99 |
| Bifid uvula | + | − | − | 1/3 | − |
| Open-mouth appearance | − | − | − | 0/3 | 80–99 |
| Wide mouth | − | − | − | 0/3 | 30–79 |
| Eversion of the lower lip | − | + | − | 1/3 | 80–99 |
| Prominent lips | + | + | + | 3/3 | 80–99 |
| Supernumerary nipples | + | + | + | 3/3 | − |
| Inverted nipples | − | + | − | 1/3 | − |
| Pectus carinatum | + | + | + | 3/3 | 80–99 |
| Delayed skeletal maturation | − | − | − | 0/3 | 80–99 |
| Short stature | + | + | + | 3/3 | 80–99 |
| Craniofacial hyperostosis | − | − | − | 0/3 | 80–99 |
| Large palms | − | − | − | 0/3 | 80–99 |
| Metacarpal pseudoepiphyses | − | − | − | 0/3 | 30–79 |
| Brachydactyly | − | − | − | 0/3 | 80–99 |
| Abnormal diaphysis morphology | − | − | − | 0/3 | 80–99 |
| Spinal abnormalities | + | + | + | 3/3 | 80–99 |
| Joint hyperflexibility | − | − | − | 0/3 | 80–99 |
| Muscular hypotonia | − | − | − | 0/3 | 80–99 |
| Exercise intolerance | − | + | − | 1/3 | − |
| Mild obstructive sleep apnea | + | − | − | 1/3 | 5–29 |
| Sensorineural hearing loss | − | − | − | 0/3 | 5–29 |
| Cardiovascular disease | − | − | − | 0/3 | 14 (Rogers & Abidi, 1993) |
- Abbreviations: +, feature present; −, feature absent.
- a “Coffin–Lowry syndrome.” Genetic and Rare Diseases Information Center, U.S. Department of Health and Human Services, rarediseases.info.nih.gov/diseases/6123/coffin-lowry-syndrome).
2.2 Individual III-2
Individual III-2 (Figure 1c) is an 11-year-old male, born full term, after an uncomplicated pregnancy. He was first evaluated by clinical genetics at the age of 9 because of his brother's history and his history of developmental delay, short stature, and dysmorphic features. A physical examination revealed downward slanting palpebral fissures, telecanthus, broad-based nose, widely spaced teeth, hypodontia, eversion of the lower lip, pectus carinatum, supernumerary nipples, inverted nipples, and broad-tapered fingers (Figure 1c and Table 1). He also has experienced some exercise intolerance. A hearing evaluation showed normal hearing in both ears, and an ophthalmology exam found pseudo-esotropia. A spinal radiograph revealed minimal levoscoliosis. At age 8 years, his height was 117.20 cm (−2.4 SD). At age 11 years, his height was 137.5 cm (10 percentile), weight was 41.70 kg (65 percentile), and head circumference was 56.0 cm (97 percentile). His intellectual disability is less severe than his older brother.
2.3 Individual III-3
Individual III-3 (Figure 1d) is a 9-year-old male, born at 37 weeks gestation after a pregnancy complicated by controlled gestational diabetes and intrauterine growth restriction. He was small for gestational age at birth and subsequently developed stridor. He was evaluated by clinical genetics at the age of 5 years because of his brothers’ and his history of developmental delay, short stature, failure to thrive, and dysmorphic features. On physical examination, he had short palpebral fissures, ptosis, broad-based nose, prominent lips, hypodontia, prominent pectus carinatum, supernumerary nipple, and broad-tapered fingers (Figure 1d and Table 1). A hearing evaluation was normal. A spinal radiograph revealed moderate neuromuscular scoliosis, which also has required bracing. He also has a history of stimulus-induced drop attacks. At age 9 years, his height is 121.0 cm (−2.4 SD), weight is 23.6 kg (−1.6 SD), and head circumference is 52.5 cm (50 percentile). Developmentally, he is able to speak in short sentences and can hold simple conversations. Cognitively, he is more advanced than his two older brothers.
3 METHODS AND MOLECULAR FINDINGS
In 2011, Individual III-1 underwent a CGH array, using the SignatureChipOS v2.0 3-plex array including 134,811 oligonucleotide probes, which was normal. In 2015, Individual III-1 had RPS6KA3 Sanger sequencing of exons 1 through 22 which was negative. In 2016, an X-linked intellectual disability next-generation sequencing panel with 114 genes also was negative for a causative variant. In 2017, whole exome sequencing of the same patient was performed at the Greenwood Genetic Center, using the Agilent SureSelectXT Clinical Research Exome Kit, on a research basis and an in-house algorithm used to detect potential deletions and duplications identified the hemizygous, likely pathogenic, duplication of exons 5 through 9 of RPS6KA3 gene (GenBank: NM_004586.2) region ChrX:20205946-20213263 (based on GRCh37/hg19 build; Figure 2a). Although the exact breakpoints and intragenic location could not be determined, the duplication of exons 5–9 was confirmed by genomic quantitative PCR. The duplication was subsequently confirmed in all three brothers by targeted high-resolution aCGH 60 K oligonucleotide array at GeneDx (Figure 2b).
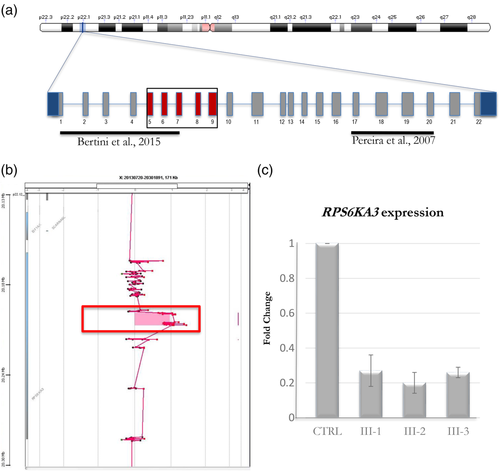
In order to assess the effect of the duplication on gene transcription, we analyzed the expression profile of RPS6KA3 by quantitative real-time PCR (qRT-PCR). Total RNA was extracted from peripheral blood cells of the patients and healthy controls using the quick-RNA Miniprep kit (Zymo Research) or Sigma Gen Elite Mammalian Total RNA Miniprep Kit. An RNA sample from the biological father and mother were not available. One microgram of total RNA was reverse-transcribed with the high capacity RNA-to-cDNA Kit (Applied Biosystems, Thermo Scientific). qRT-PCR was performed using commercial predesigned Taqman probes mapping in the nonduplicated region: RPS6KA3 Hs01078601_g1 (located in exon 21–22 boundary; Applied Biosystems, Thermofisher); ABL1 probe (Hs01104725) was used for normalization (Applied Biosystems, Thermofisher). To calculate the relative changes in gene expression, we applied the 2−ΔΔCT method as previously described (Livak & Schmittgen, 2001). The RPS6KA3 transcript in the brothers, compared with the healthy controls, showed a significant reduction in all three (Figure 2c).
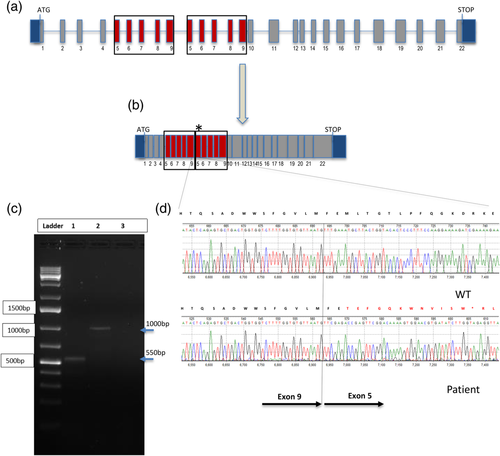
Subsequently, we analyzed the cDNA of the patients and controls by RT-PCR to determine if the duplication was present in a transcript of RPS6KA3. We designed multiple primers yielding eight overlapping PCR products covering the entire coding sequence of RPS6KA3 (data not shown). A PCR product obtained from Individual III-1 with a forward primer in exon 4 (RSK2_Ex4f 5′-CTCAGGCTCTGATGCTAGGC) and a reverse primer in exon 10 (RSK2_E10R 3′-TTTTCCTTGGAAAGGGAGTG) generated an abnormal ~1,000 bp size product (Figure 3c—Lane 2) larger than the expected 550 bp product observed in the control (Figure 3c—Lane 1), which is consistent with the introduction of 450 bp expected size of the duplication of exons 5–9 within the exons 4–10 genomic region. According to the Sanger sequencing result, the portion of cDNA comprising exon 5 to 9 is a direct tandem duplication resulting in an insertion of 450 nucleotides in the normal RPS6KA3 mRNA between nucleotide 774 and 775 and causing the frameshifting alteration p.M261Tfs13* (Figure 3d).
4 DISCUSSION
We describe three brothers with classic clinical and radiographic findings of CLS including moderate intellectual disability, broad-tapered fingers, skeletal anomalies, and characteristic facial features. Evaluating these brothers, we identified a novel intragenic duplication in the RPS6KA3 gene as the cause for their CLS features.
Previously, Newman, Hermetz, Weckselblatt, and Rudd (2015) have shown that most interstitial duplications are tandem and often lie in the proper orientation causing gene reading frame disruption and lead to loss-of-function (LOF) mutations. Two previous groups have identified direct duplications in RPS6KA3 that caused an alteration in the coding region leading to LOF either by mRNA level reduction or impacting the protein function (Bertini et al., 2015; Pereira, Heron, & Hanauer, 2007). Therefore, we hypothesize that the duplication observed in each of the three brothers presented here has impacted the RPS6KA3 gene function through one of the aforementioned mechanisms. Overall, the decreased level of RPS6KA3 transcript together with the deduced location of the duplication (Figure 3), which is predicted to disrupt the reading frame and create a premature stop codon, strongly suggest nonsense-mediated decay as the most likely mechanism explaining the LOF phenotype.
In 2007, Pereira et al. reported a patient with the typical clinical findings of CLS but who did not have a sequence-detected mutation in RPS6KA3. Subsequently, western blot analysis revealed that the RSK2 protein was much larger than expected (Pereira et al., 2007). Further molecular studies found an in-frame tandem duplication of exons 17–20, resulting in a nonfunctioning protein, which the authors postulated caused CLS. Subsequently, Bertini et al. (2015) described a male patient with a 625 kb duplication of Xp22.12, which includes only one gene, RPS6KA3. Although the exact intragenic location of the duplication was not identified, the mutation involved in tandem partial duplication of exons 1 through 7. Bertini et al. (2015) then suggested that the partial duplication inhibited the expression of the gene resulting in LOF of the protein kinase activity. The patient had mild intellectual disability, short stature, and orthodontic problems. However, his phenotype was not typical of CLS having an absence of the characteristic facial appearance (Bertini et al., 2015).
Other duplications have been reported to cause syndromic and nonsyndromic X-linked intellectual disability. In 2013, Matsumoto et al. described a family with individuals who possessed a 584 kb microduplication of Xp22.12. The microduplication contained seven genes, six of which were uncharacterized and the seventh was the RPS6KA3 gene. Molecular analyses demonstrated that the RPS6KA3 protein levels in lymphoblasts of these individuals were increased between 1.53- and 2.14-fold compared to controls. The phenotype was variable among the affected individuals but Matsumoto et al. (2013) stated that the duplication was likely causing their mild nonsyndromic intellectual disability. Other larger duplications with multiple genes, causative for nonsyndromic X-linked intellectual disability, have been described by Madrigal et al. (2007), Thorson et al. (2010), and Tejada et al. (2011).
As presented above and summarized in Table 1, all three brothers have classical characteristics of CLS including moderate intellectual disability, global developmental delay, speech delay, dental abnormalities, prominent lips, pectus carinatum, short stature, spinal abnormalities, and broad-tapered fingers. However, these siblings have other features not described as characteristic findings of CLS and include short palpebral fissures, ptosis, pseudo-esotropia, bifid uvula, and supernumerary and inverted nipples. On the other hand, other characteristics that are seen in 80–99% of individuals with CLS were not found in these brothers including prominent forehead, depressed nasal bridge, anteverted nares, open-mouth appearance, delayed skeletal maturation, craniofacial hyperostosis, large palms, brachydactyly, joint hyperflexibility, and abnormal diaphysis morphology (Table 1).
To the best of our knowledge, the novel mutation in our patients is the second intragenic microduplication reported affecting RPS6KA3 mRNA levels and resulting in LOF. The LOF in our patients appears causative for their CLS and supports the notion that microduplications in RPS6KA3 can lead to CLS by altering the coding frame of the gene.
Analyses of the intronic sequences of RPS6KA3 by UCSC genome browser (http://genome.ucsc.edu/) reveals the presence of a multitude of Alu repeats, which may be responsible for deletion/duplication events as a plausible mechanism underlying the duplication observed in our patients. The intragenic duplication described by Pereira et al. (2007), was likely mediated by Alu recombination, and although it did not shift the reading frame, the protein analysis, nonetheless, showed an aberrant partially duplicated RSK2 protein with reduced kinase activity. Therefore, our findings, along with previous published studies, Pereira et al. (2007) and Bertini et al. (2015), provide further evidence that molecular analyses capable of detecting intragenic duplications coupled with methodologies that assess mRNA or protein function, including structural transcriptional changes, might be needed for patients clinically diagnosed with CLS but in whom classic exon targeted panels failed to detect a mutation in the RPS6KA3 gene.
When sequencing analysis of RPS6KA3 does not find a pathogenic mutation in a patient suspect to have CLS, then exon-targeting deletion and duplication analyses represents valuable approaches, considering the high number of Alu repeats present within the gene. More data about the frequency of these pathogenic duplications, often missed by conventional molecular testing, are needed in order to improve the diagnostic rate and genotype–phenotype correlations in CLS. Finally, our study provides data supporting that microduplications in RPS6KA3 can cause CLS due to LOF mechanism.
ACKNOWLEDGEMENT
We express our great appreciation to the adoptive parents of the patients reported here. We could not have completed the study without their tremendous cooperation. We would also like to thank the genetic counselor, Rebecca C. Fowler, MS, CGC and the review analyst, Laura M. Sack, PhD at GeneDx for providing the confirmatory array and the array CGH profile seen in Figure 2b. We thank Cindy Skinner for sample coordination. Supported, in part, by a grant from the South Carolina Department of Disabilities and Special Needs (SCDDSN). Dedicated to the memory of Ethan Francis Schwartz, 1996–1998.




