Fragile X syndrome in a male with methylated premutation alleles and no detectable methylated full mutation alleles
Funding information National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: DK057602; National Institute of Mental Health, Grant/Award Number: MH00088936; New York State Institute for Basic Research in Developmental Disabilities; New York State Office for People with Developmental Disabilities
Abstract
Most cases of fragile X syndrome (FXS) result from aberrant methylation of the FMR1 gene. Methylation occurs when the number of tandemly arranged cytosine guanine guanine (CGG)-repeats in the 5′ end of the transcriptional unit of FMR1 exceeds a certain critical threshold, thought to be between 200 and 400 repeats. Such alleles are referred to as full mutation (FM) alleles. Premutation (PM) alleles, alleles with 55–200 repeats, are generally not aberrantly methylated and in fact may have hyperexpression of the FMR1 mRNA. We describe here a male who meets the diagnostic criteria for FXS, who is highly mosaic with a mixture of multiple PM and FM alleles and 50% methylation. However, the methylated alleles are limited to two alleles in the PM range, ~165 and ~175 repeats respectively, with the FM alleles being unmethylated. This finding has implications for FXS diagnosis as well as for efforts to delete the repeat in individuals with FXS using a CRISPR-Cas9 approach.
1 INTRODUCTION
Fragile X syndrome (FXS) is an X-linked disorder that is the most common heritable cause of intellectual disability (ID), occurring in about 1 in 4,000 males and 1 in 8,000 females. Most cases of FXS result from the inheritance of an FMR1 allele that has >200 cytosine guanine guanine (CGG)-repeats in its 5′ untranslated region (UTR) (Lozano, Rosero, & Hagerman, 2014). Such an allele, known as a full mutation (FM) allele, usually undergoes aberrant DNA methylation that results in reduced transcription. This results in a deficit of the FMR1 gene product, FMRP, a multifunctional protein that is highly expressed in brain. In females the clinical presentation is typically milder due to the protective effect of a second X chromosome (Lozano et al., 2014). Other symptoms of FXS include autistic-like behavior, anxiety, hypersensitivity to sensory stimuli, attention deficit hyperactivity disorder (ADHD), depression, and physical attributes such as a long face, large ears, and macroorchidism (Lozano et al., 2014).
FMR1 alleles with 55–200 CGG-repeats are known as premutation (PM) alleles and such alleles are often hypertranscribed but translated inefficiently rather than silenced (Tassone et al., 2000). The expanded CGG repeat is unstable and shows a propensity to expand and contract both on germ line transmission and during somatic development of the individual. As a result, upwards of 40% of individuals may be mosaic for some mixture of PM and FM alleles (Nolin, Glicksman, Houck Jr., Brown, & Dobkin, 1994) and this mosaicism may vary among tissues (Dobkin et al., 1996; Taylor et al., 1999). In addition to mosaicism for the number of repeats, FM alleles also show mosaicism for the extent of DNA methylation, with most subjects with FM alleles showing evidence of some FMR1 transcription (Tassone, Hagerman, Taylor, & Hagerman, 2001). Rare individuals are seen with little or no methylation. These individuals tend to have a normal or borderline normal intelligence quotient (IQ) (Basuta et al., 2015; Godler et al., 2011; McConkie-Rosell et al., 1993; Santa Maria et al., 2014).
We describe a male subject with a diagnosis of FXS and an unusual repeat profile. The profile shows extreme repeat size mosaicism and an unusual methylation mosaicism. Specifically, ~50% of his alleles are a diverse mixture of unmethylated PM and FM alleles, while the remaining ~50% of his alleles are PM alleles that are methylated. This finding has implications for diagnosis as well as for attempts to reverse FXS by deletion of the repeats using CRISPR-Cas9 approaches (Park et al., 2015; Xie et al., 2016).
2 MATERIALS AND METHODS
2.1 Physical and psycho-developmental assessments
The subject participated in an ongoing National Institutes of Health (NIH) study of young men with FXS (06-M-0214, NCT00362843). Consent was obtained according to the protocol, which was approved by an NIH Institutional Review Board. The subject's medical history was reviewed. A physical and psychiatric examination, as well as a neurodevelopmental evaluation including the Vineland Adaptive Behavior Scales (VABS-II), Adult Behavior Checklist (ABCL), Autism Diagnostic Observation Schedule (ADOS-2), Autism Diagnostic Interview (ADI-R), and Wechsler Adult Intelligence Scale (WAIS-IV) were conducted as part of the study.
2.2 Genetic testing
Standard cytogenetic analysis was carried out by the Biomolecular Laboratories (The Children's Mercy Hospital, Kansas City, MO). This analysis indicated a normal 46, XY male karyotype with no evidence of gross chromosomal abnormalities at the 550 band level using trypsin-Giemsa staining. Isolation of genomic DNA from blood and CVS samples was carried out according to standard procedures (Nolin et al., 2011). Isolation of genomic DNA from saliva was carried out as described previously (Hayward & Usdin, 2017). Quantitative methylation analysis of the flanking sequence of the patient's FMR1 repeat was carried out at NIH on bulk genomic DNA from saliva as previously described (Hayward, Zhou, Kumari, & Usdin, 2016). Analysis of the repeat in bulk DNA and in small-pool polymerase chain reaction (PCR) of saliva used modifications of previously published protocols (Hayward et al., 2016; Hayward & Usdin, 2017). Specifically, to prepare for PCR across the repeat, 600 ng of genomic DNA were placed in a 40 μL reaction volume containing 50 mM TrisCl pH 9, 1.5 mM MgCl2, 22 mM (NH4)2SO4 and 1 μL of FastDigest HindIII. This was divided into 2 × 20 μL aliquots and 0.5 μL of HpaII added to one aliquot. After overnight incubation at 37°C the resulting digested samples were used as templates for both bulk and small-pool PCR. For bulk PCR, 5 μL of the HindIII and HindIII/HpaII digested samples were used in a final volume of 20 μL containing 50 mM Tris HCl pH 9, 1.75 mM MgCl2, 22 mM (NH4)2SO4, 2.5 M betaine, 2% DMSO, 0.2 mM dATP and dTTP, 0.47 mM dCTP and dGTP, 0.5 μM each primer (Not_FraxC and FAM-labeled Not_FraxR4; Hayward et al., 2016), and 0.75 U KAPA2G Robust HotStart polymerase. The reaction mixes were placed in a PCR block preheated to 70°C, and samples were heated to 98°C for 3 min, followed by 32 cycles of (98°C 30 s, 59°C 30 s, 72°C 210 s) and then incubated at 72°C for 10 min. The resultant PCR products were analyzed using short run capillary electrophoresis (sample injection for 15 s at 1,500 V followed by electrophoresis at 15,000 V for c. 50 min to collect 14,961 data points). The resulting fsa files were visualized using a custom R script. The results were confirmed using an AmplideX® kit (Asuragen, Austin, TX) and Southern blotting with StB12.3 probe by means of standard procedures. The PCR profile in blood was obtained with a commercially available kit from Abbott Molecular (Chicago, IL) used according to the manufacturer's instructions. The PCR product obtained in this way was also resolved by agarose gel electrophoresis, blotted and hybridized according to standard procedures using the StB12.3 probe.
For small-pool PCR, the concentration of digested DNA was ascertained using a Denovix DS-11 spectrophotometer and serial dilutions made in the digestion buffer to generate a final solution of 15 μL containing 1,200 genomes (assuming the mass of a single genome to be 3 pg). This solution was brought to a final volume of 60 μL containing 50 mM TrisCl pH 9, 1.5 mM MgCl2, 22 mM (NH4)2SO4, 0.2% Triton X-100, 2.5 M betaine, 2% DMSO, 0.5 mM each dNTP, 0.5 μM each primer (Not_FraxC and Not_FraxR4), and 1.2 U Q5 Hot Start polymerase. This was then divided into 6 × 10 μL aliquots to give 200 genomes per PCR reaction and subjected to the same PCR protocol as for the bulk PCR except that 35 cycles were used instead of 32. PCR products were visualized using agarose gel electrophoresis followed by postelectrophoresis staining with ethidium bromide.
3 RESULTS
3.1 Clinical report
The subject is a right-handed, white male aged 20 years. His birth was without complications. He first walked at 2 years, spoke his first words at 2½, and was toilet trained at 5 years. At age two, he was seen by a pediatrician for symptoms of mild ataxia and a delay in meeting developmental milestones. He was found to be negative for Angelman syndrome and was diagnosed with FXS by Southern blotting. The subject also has a medical history of benign exertional heart murmur, mild dilatation of aortic root, anterior pectus carinatum, varicocele, a high arched hard palate and mild kyphosis. He lives with family and is employed in a special accommodation program at a local hospital. At the time of evaluation the subject was in his last year of a high school special education program. The subject has three older siblings, two of whom were diagnosed with FXS: a brother with severe ID, aggressive behavior and ADHD and a sister with mild ID, ASD, and mood disorder. The third sibling is a sister, a PM carrier with a mood disorder and ADHD. The family history is positive for some early childhood deaths of unknown etiology, multiple relatives with ID, and three cousins with FXS and one with spina bifida.
A brief psychiatric examination showed mild anxiety, shyness, restricted affect, and some repetitive behavior, although he did not meet criteria for a diagnosis of social anxiety. He was slightly clumsy, but spoke fluently. Based on the ADOS-2, ADI-R and clinical presentation, he did not meet the criteria for autism, with a total score on the ADOS-2 below the cutoff for autism based on the ADI-R diagnostic algorithm focused on symptoms at age 4–5. The subject's scores on the VABS-II, a parent report interview that measures adaptive functioning in the areas of communication, daily living, and socialization (Table 1a), were consistent with adaptive functioning in the low range, with a relative strength in socialization. The subject's scores on WAIS-IV, a standardized measure of intellectual abilities for adults aged 16–90 years (Table 1b) indicate overall cognitive functioning (full scale IQ) in the extremely low range. Most scores were consistent, both within and across index scores. Both IQ and adaptive behavior scores and clinical judgment support a diagnosis of ID in the mild severity level. The ABCL is an informant-based measure of problem behaviors for adults aged 18–59 years. Whereas the ABCL was not developed for individuals with ID, it can be a useful tool for identifying clinically meaningful areas of problem behavior and has been used in individuals with genetic conditions associated with ID (Matson, Belva, Hattier, & Matson, 2012). His scores in all domains measured were not elevated, based on his age.
| Index score | Standard score (mean = 100, SD = 15) | Percentile |
|---|---|---|
| (a) Vineland adaptive behavior scores | ||
| Communication | 57 | <1 |
| Daily living skills | 61 | <1 |
| Socialization | 68 | 2 |
| Adaptive behavior composite | 60 | <1 |
| (b) Wechsler Adult Intelligence Scale (WAIS-IV) | ||
| Verbal comprehension | 58 | 0.3 |
| Perceptual reasoning | 65 | 1.0 |
| Working memory | 53 | 0.1 |
| Processing speed | 56 | 0.2 |
| Full scale IQ | 52 | 0.1 |
3.2 Genetic analysis
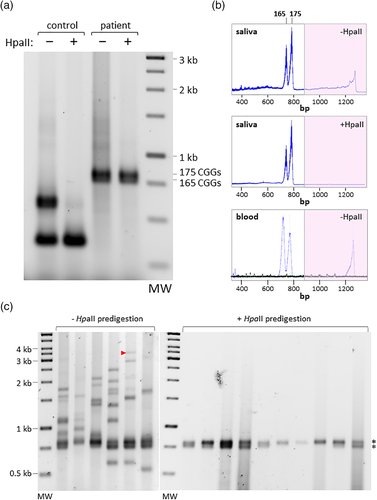
The subject shows an unusual repeat PCR profile in saliva. Specifically, on agarose gels a strong doublet corresponding to ~165 and ~175 repeats is seen superimposed on a smear of larger products (Figure 1a). The smear was much reduced when the genomic DNA was predigested with HpaII, a methylation-sensitive enzyme with a cleavage site within the PCR amplicon (Figure 1a). However, the strong PM doublets still remained, indicating that the PM alleles were methylated. When the samples were analyzed by capillary electrophoresis, the doublet was clearly apparent in both the predigested and undigested samples (Figure 1b). A smear that included material in the FM range was also apparent in the undigested sample that was missing from the predigested sample, confirming that the FM alleles were largely, perhaps completely, unmethylated. Since we have previously shown that our assay is capable of identifying a methylated allele with >900 repeats when it constitutes ~5% of the alleles in the population (Hayward et al., 2016), our data suggest that a methylated FM allele, if it exists, would likely constitute a relatively small fraction of the total alleles. The repeat PCR profile was verified using a commercially available PCR kit (AmplideX®), that also revealed an absence of adenine guanine guanine (AGG) interruptions (Supporting Information Figure S1A). Since AGG interruptions tend to stabilize the repeat tract (Latham, Coppinger, Hadd, & Nolin, 2014; Nolin et al., 2015), the absence of AGG interruptions may contribute to the extreme heterogeneity of the unmethylated alleles. A similar repeat PCR profile was also seen in blood with a different commercially available PCR assay (bottom panel of Supporting Information Figure S1B). Blotting and hybridization of the PCR product in this instance also showed no evidence of methylated FM alleles (Supporting Information Figure S1B). Furthermore, standard Southern blotting of HindIII and NruI digested genomic DNA did not reveal the presence of any methylated alleles that may have been too large to amplify by PCR (Supporting Information Figure S1C). Since the NruI sites of the 165 and 175 repeat-containing alleles are protected from digestion, methylation is not limited to the two HpaII sites interrogated in the repeat PCR assay. Small-pool PCR (SP-PCR) is a technique involving multiple PCR reactions with genomic DNA diluted such that each PCR reaction contains just a few genome equivalents for use as a template. As such, SP-PCR better reveals the extent of size heterogeneity in a population of alleles than PCR carried out on bulk DNA (Gomes-Pereira, Bidichandani, & Monckton, 2004). As can be seen in Figure 1c, all of the SP-PCR reactions generated with DNA which had not been predigested with HpaII contained one or both of the alleles that correspond in size to the methylated PM alleles seen in the bulk PCR (Figure 1a). In addition, all six PCR reactions contained multiple unique alleles ranging in size from alleles in the PM range to large FM alleles including at least one with ~1,000 repeats (indicated by the red arrowhead). In contrast, in the HpaII digested samples, the allele mixture was much less complex, with all alleles detected corresponding in size to the methylated PM alleles seen previously. These results are consistent with the idea that this patient is mosaic for hundreds, if not thousands, of alleles that include two methylated PM alleles and a large array of FM alleles that are unmethylated. Since many large FM alleles were detected, the failure to see evidence of significant levels of large methylated alleles in the bulk PCR is not due to problems with amplifying such alleles. Additionally, given that our assay is able to detect alleles with 1,000 repeats or more, even in a complex mixture of alleles (Hayward et al., 2016), it indicates that the proportion of large methylated FM alleles in this sample is likely to be small at best, with the methylated PM alleles making up the majority of the methylated alleles in the population. Since PCR amplification across the repeat may not be quantitative because of the difficulty with DNA synthesis through the repeat, we also analyzed the methylation status of the subject's alleles using a quantitative PCR assay that interrogates methylation of a region upstream of the repeat (Hayward et al., 2016). This assay showed that 46% of alleles were methylated. Since the only methylated alleles detected were the two PM alleles, these alleles likely represent close to half of the subject's alleles. Since the karyotype of this individual showed no evidence of Klinefelter syndrome, we conclude that the methylation of the PM alleles represents a repeat-mediated event rather than X inactivation.
4 DISCUSSION
To our knowledge, this is the first report of an individual who meets the criteria for FXS whose methylated alleles are predominantly, if not exclusively, in the PM range. In CRISPR-Cas9 experiments where the entire CGG-repeat tract was deleted in iPSCs, methylation was not lost in all cell lines and it was suggested that in cells that divide relatively slowly, methylation is more likely to be retained (Xie et al., 2016). This effect may be exacerbated in differentiated cells where active DNA demethylation is not very effective and where methylation is thus more likely to be clonally propagated. In light of this, the simplest interpretation of the subject's PCR profile is that he inherited a FM allele that only became methylated in a subset of embryonic cells. Then, at some point in early development, contraction of methylated FM alleles generated the two different PM alleles. Since these alleles constitute ~50% of all alleles seen in saliva and blood, this contraction likely occurred relatively early in embryonic development. As such, this contraction is likely present in multiple tissues. These alleles remained methylated and, as would be expected of methylated alleles, not prone to further expansion. In contrast, the unmethylated FM alleles retained their propensity to be unstable, generating the wide assortment of unmethylated alleles that were seen. It is possible that the allele profile in brain differs from the allele profile in blood and saliva (Taylor et al., 1999). However, if the blood, saliva and brain of this individual share an allele profile, then it may well be that methylated PM alleles contribute significantly to the patient's symptoms. A previous report described an individual with mild FXS-like symptoms but an IQ in the normal range, who also had a methylated PM allele along with a larger allele that was unmethylated (Fernandez et al., 2018). However, the clinical phenotype along with Southern blotting data suggests that the methylated allele represents a relatively minor fraction of his FMR1 alleles. A second study reported another male also with an IQ in the normal range who had an allele with 180 repeats that was unmethylated as well as a smaller, less abundant, allele with 157 repeats that was methylated (Pretto et al., 2014). This individual also had a small fraction of alleles in the FM range some of which were methylated. Since it was the smaller of the two PM alleles that was methylated, this may not be a case of methylation of PM alleles resulting from a lower methylation threshold as was originally suggested. Rather it may be that the smaller methylated PM alleles in both cases resulted from contraction of a methylated FM allele, as we suggest for the individual we describe in this report. If so, then these reports would be consistent with the demonstration using CRISPR-Cas9 that deletion of the repeats does not always lead to loss of methylation (Xie et al., 2016). In addition to ramifications for strategies aimed at deleting the repeats for therapeutic purposes, this phenomenon may have diagnostic implications as well. This case also provides an interesting addition to the list of atypical FX test findings in the context of compelling cases of FXS (Coffee et al., 2008).
ACKNOWLEDGMENT
This research was supported by the Intramural Research Programs of the NIMH (ZIA MH00088936) and the NIDDK (ZIA DK057602).
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.




