Intrafamilial variability in the clinical manifestations of mucopolysaccharidosis type II: Data from the Hunter Outcome Survey (HOS)
Abstract
Several cases of phenotypic variability among family members with mucopolysaccharidosis type II (MPS II) have been reported, but the data are limited. Data from patients enrolled in the Hunter Outcome Survey (HOS) were used to investigate intrafamilial variability in male siblings with MPS II. As of July 2015, data were available for 78 patients aged ≥5 years at last visit who had at least one affected sibling (39 sibling pairs). These patients were followed prospectively (i.e., they were alive at enrollment in HOS). The median age at the onset of signs and symptoms was the same for the elder and younger brothers (2.0 years); however, the younger brothers were typically diagnosed at a younger age than the elder brothers (median age, 2.5 and 5.1 years, respectively). Of the 39 pairs, eight pairs were classified as being discordant (the status of four or more signs and symptoms differed between the siblings); 21 pairs had one, two, or three signs and symptoms that differed between the siblings, and 10 pairs had none. Regression status of the majority of the developmental milestones studied was generally concordant among siblings. Functional classification, a measure of central nervous system involvement, was the same in 24/28 pairs, although four pairs were considered discordant as functional classification differed between the siblings. Overall, this analysis revealed similarity in the clinical manifestations of MPS II among siblings. This information should help to improve our understanding of the clinical presentation of the disease, including phenotype prediction in affected family members.
Abbreviation
-
- CNS
-
- central nervous system
-
- DSM-IV
-
- diagnostic and statistical manual of mental disorders, fourth edition
-
- ERT
-
- enzyme replacement therapy
-
- GAG
-
- glycosaminoglycan
-
- HOS
-
- Hunter Outcome Survey
-
- LSD
-
- lysosomal storage disease
-
- MPS II
-
- mucopolysaccharidosis type II
-
- P10–P90
-
- 10th–90th percentile
1 INTRODUCTION
Mucopolysaccharidosis type II (MPS II; Hunter syndrome; OMIM 309900) is a rare, X-linked, life-limiting metabolic disease. The disease has an estimated incidence of 0.6–1.3 per 100,000 Live male births (Baehner et al., 2005; Meikle, Hopwood, Clague, & Carey, 1999) and is caused by deficient activity of iduronate-2-sulfatase (EC 3.1.6.13), the lysosomal enzyme that catalyzes a step in the catabolism of glycosaminoglycans (GAGs). Progressive accumulation of GAGs in tissues and organs contributes to the multi-system and multi-organ clinical signs and symptoms of MPS II (Neufeld & Muenzer, 2001). Historically, management of MPS II has been palliative. However, disease-specific treatment in the form of intravenous enzyme replacement therapy (ERT) with recombinant iduronate-2-sulfatase (idursulfase, Elaprase®; Shire, Lexington, MA) has been available since 2006.
The clinical presentation and rate of progression of the disease are highly variable. Somatic disease manifestations are present in all patients with MPS II; however, central nervous system (CNS) involvement manifests in about two-thirds of patients (Al Sawaf, Mayatepek, & Hoffmann, 2008; Holt, Poe, & Escolar, 2011; Neufeld & Muenzer, 2001; Wraith et al., 2008). A few case reports have described variation in the clinical presentation of the disease between affected family members (Quaio et al., 2012; Tchan, Devine, & Sillence, 2011; Thurmon, DeFraites, & Anderson, 1974; Yatziv, Erickson, & Epstein, 1977). However, the data available are limited and the true extent of intrafamilial variability remains unknown.
The Hunter Outcome Survey (HOS) is a large, multicentre, observational registry that was initiated in 2005 and collects real-world, long-term data on the natural history of MPS II, and the safety and effectiveness of ERT with idursulfase (Alcalde-Martin et al., 2010; Burton & Whiteman, 2011; Burton, Guffon, Roberts, van der Ploeg, & Jones, 2010; Cohn, Morin, & Whiteman, 2013; del Toro-Riera, 2007, 2008; Jones et al., 2009, 2013; Kampmann, Beck, Morin, & Loehr, 2011; Keilmann, Nakarat, Bruce, Molter, & Malm, 2012; Link et al., 2010; Mendelsohn et al., 2010; Muenzer et al., 2011, 2017; Parini, Jones, Harmatz, Giugliani, & Mendelsohn, 2016; Wraith et al., 2008). The aims of the present analysis were to investigate the extent of intrafamilial variability in male siblings with MPS II in HOS and to gain greater insight into the factors that influence phenotypic variations between brothers.
2 MATERIALS AND METHODS
2.1 Registry design
HOS is designed to collect data on individuals with MPS II during routine patient visits and assessments (Wraith et al., 2008). Patients with MPS II who are untreated and those who are receiving treatment with idursulfase are eligible for enrollment (those receiving ERT with a product other than Elaprase are not eligible for inclusion). The registry captures a broad range of disease- and treatment-related information, both prospectively and retrospectively. Patient visits and assessments occur as deemed appropriate by the treating physician; there are no predetermined assessments in the registry. Data can be entered from patients who are either alive at enrollment (prospective patients), or deceased at enrollment (retrospective patients), if local regulations permit. Before enrollment, Institutional Review Board or Ethics Committee approval was obtained for all participating centers. Written informed consent was obtained from each patient, their parents, or legal representative. All patient information is managed in accordance with national data protection standards.
2.2 Patient population and data analysis
As of July 2015, 1,091 patients were enrolled from 124 centers in 28 countries. Of these patients, 936 were followed prospectively. Male patients who had at least one male sibling with MPS II and who were aged over 5 years at the time of their last visit were included in this analysis. If families had three male siblings enrolled, the two older siblings were included as they were followed by their physician for a longer period of time. The elder sibling in each pair was designated sibling 1. In two of the sibling pairs included in this analysis, the brothers were twins.
Demographic information was analyzed for all patients for whom data were available. Data on treatment duration and the ages at signs and symptoms onset, diagnosis, and treatment start were analysed for each sibling pair. Data on the occurrence of signs and symptoms, along with information on surgery (except bone marrow transplantation [BMT] and port placement), height, and developmental milestones were also analyzed. Height was measured according to the standard clinical practice at each center. The presence of each clinical manifestation and the achievement of key developmental milestones were determined on the basis of the answer to a yes/no question at last visit. Cognitive involvement was determined by the assessing healthcare professional on the basis of the answer to the question: “Cognitive impairment? Yes/No” for the period from birth to HOS entry and at subsequent visits (i.e., at any time): the answer to this question could have been based on clinical impression and/or standardized cognitive testing.
Functional classification was based on the clinical impression of the attending physician and recorded as “normal” (approximate intelligence quotient [IQ] >80) or “borderline” (IQ 70–80), “educable” (IQ 50–70), “trainable” (IQ 30–50), or “profound” (IQ <30) impairment. These categories correspond to, but are not identical to, the severity levels for intellectual disability described in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (American Psychiatric Association, 2000). A functional classification of “educable” corresponds to “mild” in the DSM-IV (IQ 50/55–70), “trainable” to “moderate” (IQ 34/50–50/55), and “profoundly impaired” to “severe” and “profound” severity (IQ <35/40).
Siblings were considered to be discordant if they were reported to have different functional classification categories or if the status of four or more signs and symptoms differed.
2.3 Statistical analyses
Data are presented as the median (10–90th percentile), unless stated otherwise. The correlation of age at signs and symptoms onset between sibling 1 and sibling 2 was assessed using Pearson's correlation coefficient.
3 RESULTS
3.1 Patient population
The patient population consisted of 81 prospective patients who were aged over 5 years at the time of their last visit and who had at least one male sibling with MPS II. Three families had three siblings with the disease; only the two older siblings were included. Overall, 78 patients (39 sibling pairs) were included in the analysis, of whom 68 had received idursulfase treatment.
Demographics of the sibling pairs are shown in Table 1. Generally, sibling 2 was diagnosed at a younger age than sibling 1.
| Sibling 1 (n = 39) | Sibling 2 (n = 39) | ||
|---|---|---|---|
| Age at onset of signs and symptoms, years | n | 33 | 31 |
| Median (P10, P90) | 2.0 (0.2, 4.5) | 2.0 (0.6, 5.0) | |
| Age at diagnosis, years | n | 38 | 39 |
| Median (P10, P90) | 5.1 (2.5, 17.0) | 2.5 (0.7, 12.8) | |
| Age at last visit, years | n | 39 | 39 |
| Median (P10, P90) | 18.3 (7.1, 31.1) | 14.8 (5.4, 25.4) | |
| Age at first treatment with ERT, years | n | 35 | 33 |
| Median (P10, P90) | 14.1 (3.6, 22.6) | 10.1 (2.3, 18.5) | |
| Duration of treatment with ERT, months | n | 35 | 33 |
| Median (P10, P90) | 75.5 (23.9, 125.1) | 69.6 (32.5, 110.0) | |
| Deceased | n (%) | 4 (10.3) | 3 (7.7) |
| Age at death, years | n | 4 | 3 |
| Median (P10, P90) | 19.2 (11.9, 25.3) | 20.9 (15.4, 30.6) |
- Data were available for all patients included in the analysis unless stated otherwise. Sibling 1 was the elder sibling. ERT, enzyme replacement therapy; P10, 10th percentile; P90, 90th percentile.
In five of the 39 pairs, one sibling had died; in three pairs the elder sibling had died, and in two pairs (one of which consisted of twin brothers), the younger sibling had died. In one pair, both siblings had died. Of the seven patients who had died, five had received at least one infusion of idursulfase. In the three families where the eldest sibling had died, the siblings were reported to have the same functional classification in two pairs, but this information was missing in the third pair. The causes of death recorded were cardiac arrest, cardiac failure, and not specified. In the two families where only the younger sibling died, the pairs had the same functional classification, suggesting a similar degree of CNS involvement. Further information relating to cause of death for these individuals was not available in the database. In the family where both siblings had died, the youngest was reported to have profound cognitive involvement and died of respiratory failure; the functional classification was missing for the elder sibling and the cause of death was reported as feeding intolerance and decision to discontinue support.
3.2 Signs and symptoms
The median age at signs and symptoms onset was similar in sibling 1 and sibling 2: 2.0 years (0.2, 4.5) and 2.0 years (0.6, 5.0), respectively.
The status of various clinical manifestations reported at any time was assessed in each pair (Table 2). The signs and symptoms for which the status differed most frequently were sleep apnea, behavioral problems and hyperactivity, while joint stiffness and cardiovascular signs, and symptoms differed least frequently. Of the 39 pairs, eight pairs (20.5%) were classified as being discordant (i.e., the status of four or more signs and symptoms differed between the siblings). A total of nine pairs (23.1%) were reported to have one sign or symptom that differed between siblings, six pairs (15.4%) had two differences, six pairs (15.4%) had three differences, and 10 pairs (25.6%) had no differences reported.
| Neurological | Abdominal | Audiological | Cardio-vascular | Musculo-skeletal | Pulmonary | Surgery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family number | Behavioral problems | Cognitive impairment | Functional classification at last visit | Hydro-cephalus | Hyperactivity | Seizure | Hernia | Hearing aids device | Hearing loss | Cardio-vascular | Joint stiffness | Oxygen dependent | Sleep apnea | Surgerya | Total number of discordant signs and symptomsb |
| 1 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 |
| 2 | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 4 |
| 3 | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 5 |
| 4 | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 4 |
| 5 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| 6 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| 7 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 3 |
| 8 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 9 | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 2 |
| 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | 2 |
| 11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 1 |
| 12 | No | Yes | Missing | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 3 |
| 13 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 3 |
| 14 | Yes | Yes | Missing | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 3 |
| 15 | Yes | Yes | Missing | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 1 |
| 16 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 4 |
| 17 | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 4 |
| 18 | Yes | Yes | Missing | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | 3 |
| 19 | Yes | Yes | Missing | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| 20 | No | Yes | Missing | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 3 |
| 21 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | 2 |
| 22 | Yes | Yes | Missing | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 23 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 2 |
| 24 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| 25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 26 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 1 |
| 27 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| 28 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 29 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 30 | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 4 |
| 31 | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 5 |
| 32 | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 1 |
| 33 | Yes | Yes | Missing | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 2 |
| 34 | No | Yes | Missing | No | No | Yes | No | No | No | Yes | Yes | Yes | No | No | 7 |
| 35 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 36 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 0 |
| 37 | Yes | Yes | Missing | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 38 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| 39 | Yes | Yes | Missing | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 |
| Total number of pairs with discordant classification | 11 | 3 | 4 | 6 | 10 | 7 | 4 | 7 | 4 | 2 | 2 | 7 | 13 | 3 | |
- Bold values indicate the sibling pairs for which the status of the indicated signs and symptoms differed between sibling 1 and sibling 2. Sibling 1 was the elder sibling.
- a Any surgery except bone marrow transplantation and port placement.
- b The total number of discordant signs and symptoms does not include functional classification or surgeries as these are not categorized as signs or symptoms in the Hunter Outcome Survey database.
3.3 Regression in developmental milestones
Data on regression in certain developmental milestones were assessed at any time in the sibling pairs (Supplementary Table S1). Walking regression status differed in eight pairs, speech regression status differed in eight pairs, dress alone regression status differed in one pair, and toilet training regression status differed in one pair. Only one sibling pair was discordant in more than one developmental milestone. For the majority of pairs with information available, regression status was the same in both siblings.
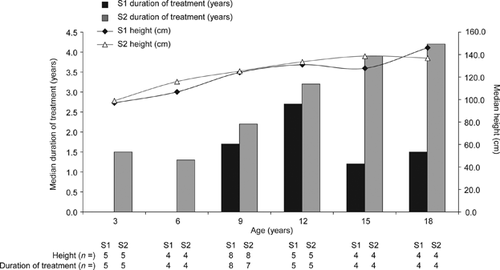
3.4 Height
Height was analyzed in the sibling pairs for whom these data were available for both brothers (Figure 1 and Supplementary Table S2). There was no significant difference in the median height of the siblings in the brother pairs, although the median duration of treatment was typically greater for sibling 2 than for sibling 1 at each time point. However, it is important to note that at each time point, data were not always from the same brother pairs.
3.5 Functional classification status
Last-reported functional classification, used as a measure of CNS involvement, was available for 28 of the 39 pairs. Functional classification was the same in both siblings in 24 pairs (85.7%). The siblings in four pairs had different functional classification and were considered to be discordant (14.3%) (Table 3).
| Sibling 2 | |||||||
|---|---|---|---|---|---|---|---|
| Normal | Borderline | Educable | Trainable | Profound | Missing | Total (sibling 1) | |
| Sibling 1 | |||||||
| Normal | 18 | 0 | 0 | 0 | 0 | 1 | 19 |
| Borderline | 1a | 1 | 0 | 1b | 0 | 1 | 4 |
| Educable | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Trainable | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Profound | 0 | 1c | 1d | 0 | 4 | 0 | 6 |
| Missing | 0 | 0 | 0 | 0 | 1 | 7 | 8 |
| Total (sibling 2) | 19 | 2 | 1 | 2 | 5 | 10 | 39 |
- Bold values indicate the number of sibling pairs for which the functional classification differed between sibling 1 and sibling 2. Sibling 1 was the elder sibling. Functional classification was collected in the database based on the clinical impression of the attending physician and classed as “normal” (approximate intelligence quotient [IQ]>80) or “borderline” (IQ 70–80), “educable” (IQ 50–70), “trainable” (IQ 30–50) or “profound” (IQ < 30) impairment.
- There were four sibling pairs in whom the functional classification was discordant between sibling 1 and sibling 2: afamily 1: sibling 1 borderline, sibling 2 normal; bfamily 31: sibling 1 borderline, sibling 2 trainable; cfamily 17: sibling 1 profound, sibling 2 borderline; dfamily 32: sibling 1 profound, sibling 2 educable.
- Functional classification differed by two or more categories in three pairs (families 17, 31 and 32).
Patient characteristics and signs and symptoms in the pairs with discordant functional classification are shown in Table 4. In family 1, the functional classification for sibling 1 was “borderline” and for sibling 2 was “normal” (sibling 1 was 28.3 years of age and sibling 2 was 23.1 years of age at last functional classification). Hearing aid device and oxygen dependence were reported only for sibling 1. The need for a hearing aid device in sibling 1 could potentially explain the difference in functional classification in this sibling pair. In family 17, sibling 1 had a functional classification of “profound” and sibling 2 was “borderline” (age at last functional classification was 14.9 years and 10.5 years for sibling 1 and sibling 2, respectively). Sibling 2 had undergone a BMT at 5.4 years of age. Seizures and joint stiffness were reported for sibling 1 only; behavioral problems and hyperactivity were reported for sibling 2 only. In family 31, the functional classification for sibling 1 was “borderline” and for sibling 2 was “trainable” (sibling 1 was 17.9 years and sibling 2 was 10.3 years at last functional classification). Hernia and joint stiffness were reported for sibling 1 only; hydrocephalus, seizure, and surgery were reported for sibling 2 only. In family 32, sibling 1 had a functional classification of “profound” and sibling 2 was “educable” (age at last functional classification was 8.4 years and 5.8 years for sibling 1 and sibling 2, respectively). Hearing loss was reported in sibling 1 only; behavioral problems and hyperactivity were reported in sibling 2 only.
| Family 1 | Family 17 | Family 31 | Family 32 | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Sibling 1 | Sibling 2 | Sibling 1 | Sibling 2 | Sibling 1 | Sibling 2 | Sibling 1 | Sibling 2 |
| Age at diagnosis, years | 2.5 | 1.5 | 4.8 | 1.3 | 4.0 | 4.0 | 3.3 | 0.8 |
| Age at last visit, years | 31.1 | 24.8 | 14.9 | 10.5 | 17.9 | 11.8 | 8.4 | 5.8 |
| Functional classificationa | Borderline | Normal | Profound | Borderline | Borderline | Trainable | Profound | Educable |
| Age at last functional classification, years | 28.3 | 23.1 | 14.9 | 10.5 | 17.9 | 10.3 | 8.4 | 5.8 |
| Idursulfase treatment | Yes | Yes | Yes | Nob | Yes | Yes | Yes | Yes |
| Age at treatment start, years | 20.1 | 15.6 | 5.2 | N/A | 14.1 | 5.1 | 3.5 | 0.9 |
| Duration of treatment, monthsc | 131.7 | 110.0 | 116.6 | N/A | 45.3 | 79.6 | 59.6 | 58.4 |
| Signs and symptoms | ||||||||
| Neurological | ||||||||
| Behavioral problems | No | No | No | Yes | No | No | No | Yes |
| Cognitive impairment | No | No | Yes | Yes | No | No | Yes | Yes |
| Hydrocephalus | No | No | No | No | No | Yes | No | No |
| Hyperactivity | No | No | No | Yes | No | No | No | Yes |
| Seizure | No | No | Yes | No | No | Yes | Yes | Yes |
| Abdominal | ||||||||
| Hernia | No | No | No | No | Yes | No | No | No |
| Audiological | ||||||||
| Hearing aids device | Yes | No | No | No | No | No | No | No |
| Hearing loss | Yes | Yes | No | No | No | No | Yes | No |
| Cardiovascular | ||||||||
| Cardiovascular | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Musculoskeletal | ||||||||
| Joint stiffness | Yes | Yes | Yes | No | Yes | No | Yes | Yes |
| Pulmonary | ||||||||
| Oxygen dependent | Yes | No | No | No | No | No | No | No |
| Sleep apnea | No | No | No | No | No | No | No | No |
| Surgeryd | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
- Bold values indicate the sibling pairs for which the status of the indicated signs and symptoms differed between sibling 1 and sibling 2. Sibling 1 was the elder sibling.
- a Of the four sibling pairs who had discordant functional classification, functional classification differed by two or more categories in three pairs (families 17, 31 and 32).
- b In family 17, sibling 2 was not treated because he had received a bone marrow transplant (BMT).
- c Duration of treatment was calculated as the time from treatment start to last visit.
- d Any surgery except BMT and port placement. N/A. not available.
Analysis of functional classification in the pairs was also performed using data when the siblings were the same age; this information was available for 16 pairs (Supplementary Table S3). Functional classification was the same in 13 pairs and differed in three pairs (three out of the four pairs described above; family 1, sibling 1 “borderline,” sibling 2 “normal;” family 17, sibling 1 “profound,” sibling 2 “borderline;” and family 32, sibling 1 “trainable,” sibling 2 “educable”). In family 31, sibling 2 was classified as “trainable” at last visit; however, data were not available for sibling 1 at the age corresponding to sibling 2.
Patient characteristics and signs and symptoms in the three pairs with discordant functional classification when the siblings were the same age are shown in Supplementary Table S4. In family 1, the data assessed were collected at age 23.0 years and 23.1 years for sibling 1 and sibling 2, respectively. At these ages, sleep apnea was recorded for sibling 1 only. In family 17, the data assessed were collected when sibling 1 was 11.0 years and sibling 2 was 10.5 years. Hydrocephalus, joint stiffness and sleep apnea were reported for sibling 1 only. In family 32, the data assessed were at age 6.0 years and 5.8 years for sibling 1 and sibling 2, respectively. Hearing aid device was reported for sibling 1 only, and hearing loss was reported for sibling 2 only.
4 DISCUSSION
Intrafamilial variability in MPS II has been reported previously in a few case studies (Quaio et al., 2012; Tchan et al., 2011; Thurmon et al., 1974; Yatziv et al., 1977). The current analysis investigated variability in clinical manifestations among male siblings with the disease in families from the HOS registry. Our study revealed similarity in the clinical presentation of MPS II in brother pairs and we hope this will contribute to our understanding of how the disease presents in siblings. It is anticipated that these results could aid phenotype prediction in affected siblings, including CNS involvement, and ultimately help to improve patient care and management in family members with the disease.
We investigated the presence of a range of signs and symptoms, including behavioral problems, hydrocephalus, hernia, hearing loss, and joint stiffness. Of the 39 sibling pairs included in this analysis, 21 pairs were reported to have one, two, or three discordant signs and symptoms, and 10 of the pairs were reported to have no discordant clinical manifestations. The pairs were generally concordant in their regression status for most of the developmental milestones investigated, and the majority of pairs (85.7%) were reported to have concordant functional classification. One brother in five pairs had died, and both brothers in one pair had died; median age at death was similar in elder and younger siblings. However, because the information available on cause of death was limited, no definitive conclusion can be drawn from these results. Taken together, these findings suggest a high degree of intrafamilial similarity in patients with MPS II.
Of the signs and symptoms investigated, sleep apnea, behavioral problems, and hyperactivity were those reported most frequently as having a different status in the sibling pairs; cardiovascular and joint stiffness differed less frequently. One potential reason for the discordance between brothers may be age, for example, signs and symptoms that develop later in life may not have been captured. Further analysis of data from discordant brother pairs revealed a general trend: the brother without the sign or symptom tended to be older at last visit than the other brother. This suggests that the occurrence of signs and symptoms is not related to chronological maturation and that age may not be the reason for discordance. Another potential reason for the discordances between siblings could be differences in treatment patterns. Overall, the median duration of ERT with idursulfase was similar for the elder and younger siblings; however, the median age at treatment start was younger for sibling 2 than for sibling 1. For the eight brother pairs who were considered to be discordant with respect to signs and symptoms (the status of four or more signs and symptoms differed between the sibling pairs), possible differences in age at treatment start and treatment duration were investigated further, however, this did not appear to explain these differences. It is possible that environmental factors may play a role.
Height of the siblings was also evaluated in this analysis. The younger siblings were generally taller than the elder siblings at all time points except at 18 years of age; however, it is important to note that this was not a longitudinal analysis and not all patients had data available at each time point. In addition, there are some potential limitations with assessment of height in patients with MPS II. For example, the accuracy of height measurements may be affected by features of the disease such as musculoskeletal abnormalities (including joint stiffness) and behavioral difficulties (Parini et al., 2016). ERT has been shown to have a positive effect on growth rate in patients with MPS II, particularly if initiated before 10 years of age (Jones et al., 2013; Schulze-Frenking, Jones, Roberts, Beck, & Wraith, 2011), and treatment duration tended to be greater for the younger siblings at the given time points. However, additional analyses, including availability of idursulfase, would be required to determine whether the increase in height was associated with ERT.
Functional classification was recorded in the database using one of five categories to identify different levels of cognitive impairment and CNS involvement. Functional classification was reported to be the same in the majority of the brother pairs. However four pairs had different functional classifications recorded and were considered to be discordant. Of these four pairs, three had functional classifications that differed by two or more categories. To rule out age as a cause for this discrepancy, functional classification of the siblings when the brothers were the same age was evaluated. Discordances were revealed in three pairs; only one had functional classifications that differed by two or more categories (family 17; brother 1 “profound,” brother 2 “borderline”). In this family, the younger brother did not receive ERT but instead had undergone a BMT at 5.4 years of age. It is possible that the difference in treatment between the two brothers in family 17 may explain the discrepancy in their reported functional classifications. However, this would require further investigation, particularly as there are conflicting reports on the impact of BMT on cognitive symptoms (Guffon, Bertrand, Forest, Fouilhoux, & Froissart, 2009; Kubaski et al., 2017; Muenzer et al., 2009). Nonetheless, it is important to note that the pairs in our analysis were generally concordant for CNS involvement, indicating that the presence of this manifestation does not typically differ between siblings.
Intrafamilial variability has been described for other lysosomal storage diseases (LSDs) such as Fabry disease, Pompe disease, and Sanfilippo syndrome (Papadopoulos, Papadimus, Michelakakis, Kararizou, & Manta, 2014; Rigoldi et al., 2014; van de Kamp, Niermeijer, von Figura, & Giesberts, 1981; Verovnik, Benko, Vujkovac, & Linthorst, 2004). It has been suggested that genetic and epigenetic factors may affect phenotypic variability in patients with LSDs, particularly for less-severe, later-onset disease (Gieselmann, 2005). However, additional studies are required to investigate this further. Clinical variability among family members with MPS II has been reported previously in a few small case studies (Quaio et al., 2012; Tchan et al., 2011; Thurmon et al., 1974; Yatziv et al., 1977). In particular, significant clinical heterogeneity was observed among seven individuals with MPS II within one family who had varying degrees of disease severity, although disease presentation was more similar between the siblings than between the other family members (Thurmon et al., 1974). Other case studies have also described variability in the severity of somatic and neurological signs and symptoms in family members with the disease (Quaio et al., 2012; Tchan et al., 2011; Yatziv et al., 1977). However, the results of our analysis suggest that this is not a common feature of MPS II. This is important information that could aid genetic counseling of affected families and help physicians decide on the best treatment for their patients.
It is important to discuss the potential limitations of this study. Unlike a clinical trial, patient enrollment in HOS is at the discretion of participating physicians, and information entered into the database is collected during routine clinical practice (Muenzer et al., 2017). In addition, regional variation in standards of care and resources may mean that there are differences in the clinical assessments performed for each patient and the frequency of follow-up visits. It should also be noted that the functional classification reported in the HOS database is not based on a standardized approach given the observational nature of the registry. Formal, in-depth, assessments of cognitive function may be performed in some clinics, however the physician may instead make an assessment on the basis of their clinical judgement during a clinic visit. As a result, this measure is only really suitable as an indicator of CNS involvement and not as a method for accurately determining the degree of cognitive impairment or disease severity. Although there is currently no standard method for assessing the cognitive aspects of MPS II (Shapiro et al., 2016), a set of recommendations for evaluating cognitive and adaptive function in patients with mucopolysaccharidoses was recently published (van der Lee et al., 2017). It is hoped that these recommendations should help to standardize the assessment of CNS involvement in patients with MPSs.
Despite these limitations, registry data play an important role in providing insights into the natural history of diseases and long-term treatment effects. This analysis of data from HOS indicates that there is similarity in the clinical presentation of MPS II among siblings. Our findings suggest that intrafamilial variability may be less common in MPS II than in other LSDs such as Fabry disease, although further investigation is required. If there is intrafamilial variability, it will be important to establish the influence of genetic factors, such as polymorphisms in modifier genes and epigenetic factors, as well as environmental factors. In conclusion, this information increases our understanding of the clinical presentation of MPS II in affected families, which in turn should help to inform physician decisions for patient care, genetic counseling, and disease management.
ACKNOWLEDGMENTS
The authors would like to thank all those involved in HOS for their valuable contributions, in particular the patients enrolled in HOS and their families, as well as the HOS Investigators and study coordinators. The authors would also like to thank the Pierfranco e Luisa Mariani Foundation, Milano, Italy, for its continued financial support for clinical assistance to metabolic patients in Monza (Dr Parini). Dr Harmatz has received funding from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through UCSF-CTSI grant number UL1 TR000004. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This study was sponsored and funded by Shire, Lexington, MA. Data collection and analyses are supported by Shire. No honoraria, grants or other forms of payment were made to the authors for the writing of the manuscript. Medical writing support was provided by Dr Gillian Quigley, Oxford PharmaGenesis, Oxford, UK, and was funded by Shire, Zug, Switzerland. The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
CONFLICTS OF INTEREST
Can Ficicioglu has provided consulting support to, and received grant support and honoraria for speaking engagements from Abbott Laboratories, Alexion, BioMarin, Hyperion Therapeutics, Inc., Pfizer, Sanofi Genzyme, Shire, Swedish Orphan Biovitrum Sobi. Roberto Giugliani has received travel grants from Actelion Pharmaceuticals Ltd, BioMarin, Sanofi Genzyme and Shire, research grants from Actelion Pharmaceuticals Ltd, Alexion, Amicus Therapeutics, BioMarin, Sanofi Genzyme and Shire, and honoraria for speaking engagements from Actelion Pharmaceuticals Ltd, Alexion, BioMarin, Sanofi Genzyme and Shire. Paul Harmatz has provided consulting support to and received grant support and honoraria for speaking engagements from ArmaGen, Alexion/Enobia, BioMarin, Chiesi, Fondazione Telethon, Inventiva Pharma, PTC Therapeutics, Inc., RegenXbio, Sangamo, Sanofi Genzyme, and Shire. Nancy J. Mendelsohn is engaged in ongoing research projects with BioMarin and Shire. Virginie Jego is an employee of Cytel, Inc. and has received consulting fees from Shire. Rossella Parini has received travel grants from BioMarin, Sanofi Genzyme, Shire, and Swedish Orphan Biovitrum (Sobi), research grants from Shire, and honoraria for speaking engagements from BioMarin, Sanofi Genzyme, and Shire.