Whole exome sequencing reveals a mutation in ARMC9 as a cause of mental retardation, ptosis, and polydactyly
Abstract
Intellectual disability (ID) refers to deficits in mental abilities, social behavior, and motor skills to perform activities of daily living as compared to peers. Numerous genetic and environmental factors may be responsible for ID. We report on elucidation of molecular basis for syndromic ID associated with ptosis, polydactyly, and MRI features suggestive of Joubert syndrome using homozygosity mapping followed by exome sequencing. The analysis revealed a novel synonymous variation p.T293T (c.879G>A) which leads to a splicing defect in ARMC9 gene. The variant is present in conserved region of ARM domain of ARMC9 protein, which is predicted to form a platform for protein interaction. This domain is likely to be altered in patient due to splicing defect caused by this synonymous variation. Our report of variant in ARMC9 Leading to Joubert syndrome phenotype (JS30), elucidates the genetic heterogeneity of Joubert syndrome, and expands the gene list for ciliopathies.
1 INTRODUCTION
Intellectual disability (ID) has a prevalence of 1–3% in population and can result from heterogeneous causes like environmental/nutritional effect, chromosomal or monogenic causes (Maulik, Mascarenhas, Mathers, Dua, & Saxena, 2011). More than 230 genes have been reported to be involved in causation of syndromes of intellectual disability. Mental retardation, ptosis, and polydactyly are a distinctive combination of clinical features reported by us. This is a rare type of intellectual disability syndrome, reported only in a single consanguineous family from India, where three individuals were affected (Panigrahi, Phadke, & Agarwal, 2002).
ID, short stature, and polydactyly was suggestive of possibility of Bardet Biedl syndrome (BBS, MIM: 209900), but absence of obesity, renal abnormality, retinopathy, and normal sexual development were some of the phenotypes that were different from BBS. Another possibility was 3MC syndrome, which was formally known as Carnevale syndrome (MIM: 265050), however patients did not had hip dysplasia, cryptorchidism, and abdominal muscle defect. For identification of candidate gene in this family we have employed homozygosity mapping followed by exome sequencing in all the three affected siblings with mental retardation, ptosis, and polydactyly phenotype.
2 MATERIALS AND METHODS
2.1 Clinical report
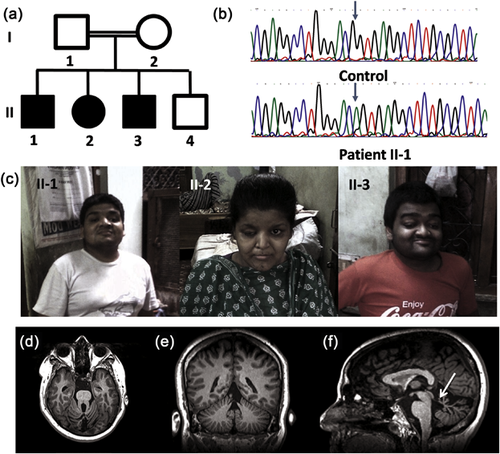
We described the clinical features in three affected siblings born out of consanguineous union, characterized as mental retardation, ptosis, and polydactyly phenotype (Panigrahi et al., 2002). The proband showed severe intellectual disability (ID), bilateral ptosis, downslanting palpebral fissures, hypertelorism, round face, high arched palate, clinodactyly, tapering of fingers, and hypermobile patellae (Figure 1c). He also had joint laxity in the metacarpophalangeal and interphalangeal joints in hands and short stature. Female sibling was also proportionately short with stocky build. She had moderate ID, ptosis in right eye, pointed chin, bilateral post axial polydactyly with joint laxity. The third male sibling had marked joint laxity similar to proband, moderate ID, bilateral ptosis, and bilateral post axial polydactyly (Table 1). We collected blood samples from all affected siblings and both parents after informed consent. This study was approved by the Institutional Ethics Committee.
| Phenotype | II-1 | II-2 | II-3 | JS30 (11 patients) |
|---|---|---|---|---|
| Intellectual disability | Severe | Moderate | Severe | 11/11 |
| Ptosis | Bilateral | Right eye | Bilateral | 7/11 |
| Polydactyly (hand) | − | + | − | 0/11 |
| Polydactyly (feet) | − | + | + | 2/11 |
| Clinodactyly | + | − | − | 0/11 |
| Downslanting palpebral fissure | + | + | + | NA |
| Short stature | + | + | + | NA |
| Stocky build | NA | + | NA | NA |
| Pointed chin | − | + | − | NA |
| Hypertelorism | + | NA | − | NA |
| Hypermobile patellae | + | NA | NA | NA |
| Joint laxity in metacarpophalangeal and interphalangeal joint | + | + | + | NA |
| Round face | + | − | + | NA |
| Tapering fingers | + | NA | − | NA |
| Flat feet | − | + | − | NA |
| Molar tooth sign | + | + | + | 11/11 |
| Vermis hypoplasia | + | + | + | 11/11 |
| Other features | Abnormal eye movement Apnea Breathing abnormality Retinal dystrophy Seizures |
2.2 Genetic analysis
Genomic DNA from all affected and unaffected family members was extracted from whole blood. SNP array analysis in all three affected individuals was performed by using Illumina Human-CytoSNP-12 v.2.1 DNA Analysis BeadChip Kit (Illumina Inc., San Diego, CA). Analysis of array data was performed using Genome studio software. Size threshold for analysis was kept as 200 kb for deletion, 500 kb for duplication, and 1 Mb for homozygous regions. Genomic Oligoarray and SNP evaluation tool (v3.0) (http://firefly.ccs.miami.edu/cgi-bin/ROH/ROH_analysis_tool.cgi) was used for detection of known disease causing OMIM genes in the common homozygous regions.
Exome sequencing was performed in all three affected sibs. Agilent SureSelectXT V5 exome capture kit (Agilent Technologies, Santa Clara, CA) was used for preparation of exome library by using ∼3 μg of genomic DNA. Exome library was sequenced to mean 100× coverage on Illumina HiSeq2000 sequencing platform. Alignment to human reference genome (GRCh37/hg19) and variant calling were performed using NEXTGENe v.2.3.4.4 (SoftGenetics, LLC, State College, PA) followed by variant annotation by ANNOVAR (Wang, Li, & Hakonarson, 2010). Variants with minor allele frequency of >0.01 in 1000 Genome project (http://www.1000genomes.org/), EVS (http://evs.gs.washington.edu/EVS/), ExAC databases (http://exac.broadinstitue.org/), and in-house database were excluded from the study. Only nonsense, non-synonymous, splice site variants, synonymous splice site variants, insertion, and deletion variants that affect coding regions of gene were used for interpretation. PCR was performed using gene specific primer set (listed in Supplementary Table S1) followed by Sanger sequencing on ABI3130 Genetic analyzer (Life Technologies, Carlsbad, CA) following the manufacturer's protocol in control, parents, unaffected sibs, and affected sibs to validate variants identified on exome sequencing.
2.3 In vivo analysis of variant at splice site
An observed variation was evaluated for splicing defect using pCAS2 minigene construct (Tournier et al., 2008). Wild-Type (ARMC9_Wt) and mutant (ARMC9_Mt) constructs were created by PCR amplification from control and patient sample, respectively, followed by cloning them in BamH I and Mlu I restriction site of pCAS2 vector. Recombinant constructs as well as pCAS2 (as control) were transfected into COS7 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cells were harvested after 18 hr of transfection and total RNA was extracted from each transfectant using RNeasy Mini Kit (Qiagen, Hilden, Germany), as per the manufacturer's instruction. Total RNA was subjected to RT PCR using SuperScript III (Invitrogen) followed by plasmid specific amplification (P1 and P2 primer) and evaluated on 2% agarose gel. Appropriate bands were purified using QIAquick Gel Extraction Kit (Qiagen) and subjected to Sanger sequencing using on ABI3130 Genetic analyzer (Life Technologies).
2.4 Molecular modelling
To analyze conservation pattern of ARMC9 (NP_001278585), protein sequences from various species were aligned. Multiple sequence alignment was performed for the region of ARM domain I, which is expected to be affected during splicing event using ClustalW software (http://embnet.vital-it.ch/software/ClustalW.html). An ARMC9 wild-type (1–665 aa) and ARMC9 mutant structural model was generated using I-TASSER software (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) for comparison of predicted molecular structures (Roy, Kucukural, & Zhang, 2010; Yang et al., 2015; Zhang, 2008).
3 RESULTS
3.1 Homozygosity mapping and exome sequencing
We did not identify any significant copy number variations in any of the affected siblings. Regions of loss of heterozygosity (LOH) greater than 1 MB shared by affected siblings were identified on chromosome 2, 7, 10, and 14 (Supplementary Table S2), harbouring 228 Refseq genes and including 81 OMIM genes. The largest region of shared homozygosity was on chromosome 2q of 11 Mb, harbouring 141 genes. None of the genes in the shared regions were found to be associated with intellectual disability, ptosis, and polydactyly occurring together. Whole exome sequencing was performed for all three affected siblings. Mapping and alignment to human reference genome was performed using NEXTGENe v.2.3.4.4 software. Variants were filtered for minor allele frequency (MAF) <0.01 in 1000 Genome project, EVS, ExAC, and in-house database of 50 Indian exomes. Mutation databases such as HGMD (http://www.biobase-international.com/products/hgmd) and Clinvar (http://www.ncbi.nlm.nih.gov/clinvar/) were also checked for presence of reported pathogenic variants in affected individuals. After filtering of variants, two candidate variants (c.654A>C in UGT1A7 and c.879G>A in ARMC9) present in shared homozygous region of 11 Mb on Chromosome 2 were studied further (Table 2 and Supplementary Table S3).
| S. No. | Gene | Position | Variant | Protein variant | Gene Function |
|---|---|---|---|---|---|
| 1 | ARMC9 | Chr2: 232104754 | c.879G>A | p.Thr293Thr | Armadillo repeat containing 9: May be involved in microtubule dynamics |
| 2 | UGT1A7 | Chr2: 234591237 | c.654A>C | p.Leu218Phe | UDP Glucuronosyltransferase family 1 member A7: Enzyme of Glucuronidation pathway |
| 3 | ZFPM1 | Chr16: 88599697 | c.1331_1339delinsCCC | p.Phe446_449Leu | Zinc finger protein Multitype 1: Partner of hematopoietic transcription factor GATA |
The variant in UGT1A7 was predicted to be pathogenic but the function of UGT1A7 gene in glucuronidation pathway did not appear to be related with the phenotype in the affected siblings. The variant in ZFPM1 was not present in shared region of Homozygosity in the patients and thus were not opted for further study. The variant ARMC9 c.879G>A, on chromosome 2q37.1, was found to be homozygous in all affected siblings, present in shared homozygous region, absent in 1000 Genome database, EVS, ExAC, in-house database of 50 exomes, and predicted to be damaging by various pathogenicity prediction software. This variant is a synonymous variant (p.T293T) in the last base of exon 9 of ARMC9 gene and was predicted to affect splicing by splice site prediction tools such as Human Splice Finder and MutPred Splice (Desmet et al., 2009; Mort et al., 2014). Sanger sequencing confirmed presence of homozygous c.879G>A variant in all three affected siblings (Figure 1b). The variant was found to be heterozygous in both parents and absent in unaffected sibling and was consistent with autosomal recessive inheritance (Figure 1a).
3.2 Characterization of variant by minigene assay for splicing defect
Since ARMC9 is not expressed in blood, in vivo characterization of this variant was done to determine whether it causes a defect in mRNA splicing using pCAS2 minigene. PCR amplification using pCAS2 minigene specific primers (P1 and P2) from cDNA prepared using all transfectants were checked on 2% agarose gel, which revealed presence of 255 bp band in PCAS (control plasmid) and ARMC9_Mt whereas ARMC9_Wt had 354 bp fragment (Figure 2).
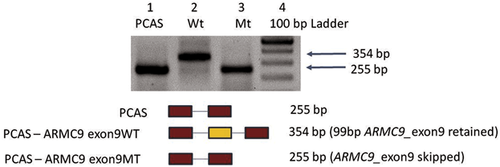
These results indicate that the variant is likely to lead to skipping of exon during splicing event. To confirm the results from RNA assay, sequencing of specific bands form RT-PCR for ARMC9_pCAS_Wt and ARMC9_pCAS_Mt was done by Sanger sequencing, which revealed deletion of 99 base pairs of exon 9 of ARMC9 gene (Supplementary Figure S1). This result confirms that synonymous variant c.879G>A (p.T293T) leads to abolition of splice site and leads to skipping of exon 9 of ARMC9 which may result in in-frame deletion of 33 amino acids (exon 9) form ARMC9 in patients.
Multiple sequence alignment of ARMC9 across vertebrates and invertebrates shows that ARM domains are highly conserved across species. Structural modelling of ARMC9 predicts similar structure to other ARM domain containing proteins such as beta-catanin, which shows presence of superheilx of alpha helices with three helices per unit, this forms a grove, which presumably may interact with its partners. ARMC9 mutant structure indicates deletion of 261–293 aa residues from wild-type ARMC9 critically disrupts its structure and hence may abolish native function of ARMC9 (Figure 3).

4 DISCUSSION
We elucidated the molecular basis in three siblings who were diagnosed with mental retardation, ptosis, and polydactyly phenotype, using homozygosity mapping and whole exome sequencing. Whole exome sequencing analysis revealed a homozygous synonymous splice site variant in ARMC9 gene (c.879G>A) in these patients. This variant leads to abolition of splice site, which leads to skipping of exon 9 during splicing event.
Intellectual disability in combination with other clinical features represents a heterogeneous group of genetic disorders with large number of causal genes (Vissers, Gilissen, & Veltman, 2016). Combination of homozygosity mapping and exome sequencing has allowed us to examine small family with few affected individuals, particularly in the case of recessive mode of inheritance and consanguinity, to efficiently limit the number of candidate genes to be studied and establish new disease-gene association.
ARMC9 was first reported in cDNA analysis of human fetal whole brain, which revealed expression of several new long coding transcripts. One of them was ARMC9, which was reported as KIA1868 (Nagase, Nakayama, Nakajima, Kikuno, & Ohara, 2001). ARMC9 is also known as Lish domain containing protein ARMC9, NS21, and Melanoma/melanocyte specific tumor antigen KU-MEL-1 (Kiniwa et al., 2001). ARMC9 is a lesser known protein, containing N-terminal LisH (Lissencephaly type 1like homology) domain and C-terminal ARM (Armadillo repeats) motifs. Proteins containing LisH domains are known to be involved in microtubule dynamics and cell division (Gerlitz, Darhin, Giorgio, Franco, & Reiner, 2005). Armadillo (ARM) repeats are characterized as repeating, 42 amino acid motifs, composed of three α helices. Although ARM repeat containing proteins do not share sequence similarity, they share a similar structure (Tewari, Bailes, Bunting, & Coates, 2010). Multiple ARM repeats fold together to form a super-helix structure, which acts as platform for interaction with protein partners. ARM containing proteins are conserved through eukaryotes and are involved in diverse cellular functions such as signal transduction, cell migration, and proliferation and cytoskeletal regulation (Coates, 2003; Valenta, Hausmann, & Basler, 2012).
We proceeded further to investigate ARMC9 function based on homology. We could not detect clear homolog but BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM) and InterProScan (Zdobnov & Apweiler, 2001) protein domain homology searches showed that there are two predicted ARM domains (154–341 and 375–584) at the C-terminus, in addition to N-Terminal Lish domain. The tandem ARM repeat domains of ARMC9 may fold together as a series of tandem helices forming a super-helix that creates a surface or groove for protein interaction similar to that of the Beta catenin (CTNNB1) ARM repeat structure. Yeast two hybrid assay has shown that ARMC9 interacts with Siah E3 ubiquitin protein ligase 1 (SIAH1) and CKLF like MARVEL transmembrane domain containing 5 (CMTM5) (Rolland et al., 2014), which indicates that ARMC9 may be involved in ubiquitination pathway like ARMC8 (Tomaru et al., 2010).
To study effect of mutation in splicing, the best method is analysis of patient RNA for respective gene, but ARMC9 has little or no expression in adult tissue (Kiniwa et al., 2001). Thus we proceeded with alternate method for assay of splicing defect using PCAS2 miningene system. Synonymous splicing defect variant, which leads to skipping of exon 9 in ARMC9 gene will lead to in-frame deletion of few ARM repeats in ARM domain (deletion of 261–293 aa), that is likely to influence protein binding capabilities of ARMC9.
It is known that armadillo repeat containing proteins can have more than one function in cells, potentially interacting with different protein partners (Onoufriadis et al., 2014) Several studies on another ARM contacting protein Beta catenin (CTNNB1) indicate that pathogenic variations in CTNNB1 can lead to wide range of neurodevelopmental disorders (ID with syndromic features) (Ligt et al., 2012; Winczewska-Wiktor et al., 2016). Studies on mouse mutant that carries mutation in armadillo repeat of Beta catenin (CTNNB1) demonstrate reduced affinity for membrane associated cadherins (Tucci et al., 2014). Similarly mutation in APC2, an ARM containing protein involved in neural development, is associated with Sotos syndrome 3 (MIM: 61719) involving ID, hyperactive behavior with macrocephaly (Almuriekhi et al., 2015). Thus ARMC9 also is likely to be involved in neurodevelopmental process and its perturbation may lead to syndromic ID.
Recent study revealed mutation in ARMC9 Leads to Joubert syndrome 30 (JS30; MIM:617622) in human and ciliopathy features in Zebrafish (Weghe et al., 2017). The study shows that ARMC9 Localizes to basal body and may be involved in ciliary machinery. In Zebrafish CRISPR-engineered armc9 shows reduction of cilia in brain ventricles, curved body, and coloboma similar to other ciliopathy associated genes. Further investigation on our patients also showed molar tooth sign and absence of cerebellar vermis, key phenotypes of Joubert Syndrome (Figure 1d–f).
In summary, here we report on a synonymous splice site variant in ARMC9 as a cause of Joubert syndrome in the family. The variant affects conserved ARM repeats at the C terminus and is likely to disrupt its interaction with other protein partners. ARMC9 joins an important group of highly conserved ARM repeat containing proteins associated with intellectual disability which includes Beta Catenin (CTNNB1), APC2. Revelation of mutation in ARMC9 Leads to JS30, extends the genetic heterogeneity of JS and expands gene list for ciliopathies. The exact nature and essential role of ARMC9 remains to be fully characterized, however these results further expand our understanding of molecular genetic basis of intellectual disability and facilitate better counseling of patients.
ACKNOWLEDGMENTS
We are grateful to the family for their support during this study. We acknowledge MedGenome (Cochin, Kerala, India) for performing exome sequencing. We also acknowledge the funding support from Department of Biotechnology, Government of India, Grant Number: BT/PR3193/MED/12/521/2011 and Indian Council of Medical Research (BMS 54/2/2013).
CONFLICTS OF INTEREST
The authors declare no conflict of interests.
WEB RESOURCES
1000 Genomes http://www.1000genomes.org/
Exome Variant Server http://evs.gs.washington.edu/EVS/
ExAC http://exac.broadinstitue.org/
dbSNP http://www.ncbi.nlm.nih.gov/SNP/
ClinVar http://www.ncbi.nlm.nih.gov/clinvar/
OMIM http://www.omim.org/
HGMD http://www.biobase-international.com/products/hgmd
Integrative Genomics Viewer (IGV) http://www.broadinstitute.org/igv/
Polyphen2 http://genetics.bwh.harvard.edu/pph2/