Discordant fetal phenotype of hypophosphatasia in two siblings
Abstract
Hypophosphatasia (HPP) is an autosomal recessive metabolic disorder with impaired bone mineralization due to mutations in the ALPL gene. The genotype-phenotype correlation of this disorder has been widely described. Here, we present two affected siblings, whose fetal phenotypes were discordant. A 31-year-old Japanese woman, G0P0, was referred to our institution because of fetal micromelia. After obstetric counseling, the pregnancy was terminated at 21 weeks’ gestation. Post-mortem radiographs demonstrated severely defective mineralization of the skeleton. The calvarial, spinal, and tubular bones were mostly missing. Only the occipital bones, mandible, clavicles, ribs, one thoracic vertebra, ilia, and tibia were relatively well ossified. The radiological findings suggested lethal HPP. Genetic testing for genomic DNA extracted from the umbilical cord identified compound heterozygous mutations in the ALPL gene (c.532T>C, p.Y178H; c.1559delT, p.Leu520Argfs*86). c.532T>C was a novel variant showing no residual activity of the protein by the functional analysis. The parents were heterozygous carriers. In the next pregnancy, biometric values on fetal ultrasonography at 20 and 26 weeks’ gestation were normal. At 34 weeks, however, a small chest and shortening of distal long bones came to attention. The neonate delivered at 41 weeks showed serum ALP of <5U/L. Radiological examination showed only mild thoracic hypoplasia and metaphyseal mineralization defects of the long bones. ALP replacement therapy was introduced shortly after birth, and the neonate was discharged at day 22 without respiratory distress. Awareness of discordant fetal phenotypes in siblings with HPP precludes a diagnostic error, and enables early medical intervention to mildly affected neonates.
1 INTRODUCTION
Hypophosphatasia (HPP) is a rare skeletal dysplasia characterized by low serum and tissue alkaline phosphatase and impaired bone mineralization (Fraser, Yendt, & Christie, 1955; Whyte, 2016). This disorder is caused by mutations and decreased activity in the ALPL gene that codes tissue nonspecific alkaline phosphatase (TNALP) (Mornet, 2000; Mumm et al., 2002), and is currently classified into five clinical types (perinatal, infantile, childhood, adult, and odonto types) (Whyte, 2016). The clinical severity of HPP often depends on the age of onset, and severe forms of HPP (i.e., perinatal lethal and infantile type) are inherited in an autosomal recessive fashion (Mornet, 2000). Genotype–phenotype correlations have been described for HPP, and the same gene mutations have been linked to comparable clinical phenotype, especially in the life-threatening type (Zurutuza et al., 1999). We, herein, present a case of two siblings with the same autosomal recessive mutations showing discordant HPP phenotype in the fetal period.
2 CLINICAL REPORT
2.1 Sibling A
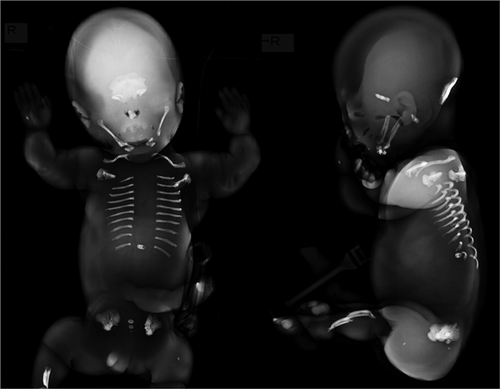
A 31-year-old woman, gravida 0, para 0, was referred to our institution because of fetal abnormal extremities. Ultrasound examination at 20 weeks of gestation showed shortening (−5.0 SD below the mean) and subjectively thin with slightly irregular contours of the major long bones. Mineralization of the caput, spine, and extremities were also markedly reduced, although polyhydramnios and small chest was absent. These findings were consistent with a lethal skeletal dysplasia, and the parents requested to terminate the pregnancy after counseling. At 21 weeks of gestation, termination of pregnancy was carried out and the baby weighing 370 g was stillborn. Post-mortem radiographs demonstrated severely defective mineralization of the skeleton. The calvarial, spinal, and tubular bones were mostly missing. Only the occipital bones, mandible, clavicles, ribs, a single thoracic vertebrae, ilia, and tibiae were relatively ossified. The radiological findings suggested perinatal lethal HPP (Figure 1). Genetic testing of genomic DNA extracted from the umbilical cord identified compound heterozygous variants in the ALPL gene, c.532T>C, p.Y178H in the Exon 6 and c.1559delT, p.Leu520Argfs*86 in the Exon 12. Each parent carries one variant allele: c.532T>C for the mother and c.1559delT for the father. Both of the parents did not have missing teeth or fragile fractures or any other signs of HPP. The levels of serum alkaline phosphatase activity were lower than the normal rage in the mother (73 U/L, normal 100–320), and at the lower end of the normal range in the father (118 U/L, normal 100–320). There was no family history indicating HPP.
2.2 Sibling B
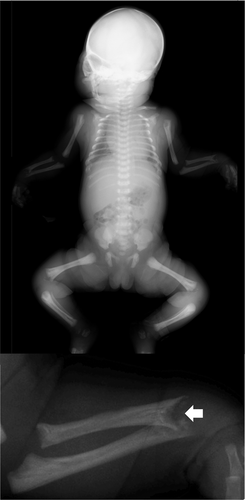
Four months after the selected termination of pregnancy for sibling A, the mother became pregnant with the same partner. Because the fetus showed normal growth trajectory in the second trimester, the parents did not request amniocentesis for prenatal diagnosis of HPP. Fetal ultrasonography at 20 and 26 weeks of gestation showed normal fetal biometry including long bones. However, shortening of radius, ulna, fibula, and small chest was noted at 34 weeks, and exacerbated at 40 weeks of gestation (ulna −4.2 SD, radius −4.4 SD, fibula −2.5 SD, and thoracic circumference −3.0 SD) (see Supplement table S1). Humerus and femur length were at the lower limit of the normal range. At 41 weeks of gestation, a female (birth weight 3,458 g; height 49.4 cm) was vaginally delivered. The neonate showed convulsion on day 0, although no signs of respiratory disorder was noted. Neonatal serum ALP was extremely low (<5 U/L). Radiological examination showed only mild thoracic hypoplasia and metaphyseal mineralization defects of the long bones. (Figure 2) Taken together, the diagnosis of HPP was made and replacement therapy of recombinant ALP (asfotase alfa, Strensiq®) was initiated shortly after birth. The ALPL gene analysis of the sibling B confirmed the same variants as the sibling A. The sibling B was discharged at postnatal day 22.
2.3 Activity of TNALP with the sequence variations
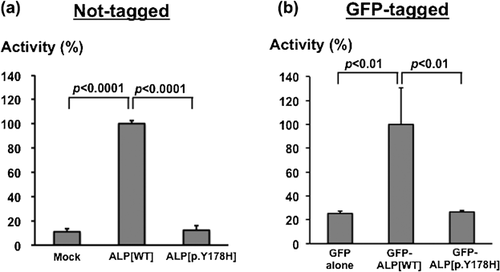
Both siblings had c.532T>C, p.Y178H and c.1559delT, p.Leu520Argfs*86 in the ALPL gene. c.532T>C was not reported previously. We evaluated an enzymatic activity of TNALP with p.Y178H using in vitro functional assay reported by Cai et al. (1998). The assay revealed that TNALP with p.Y178H had no residual activity (Figure 3). Western blotting using anti-GFP antibody (Roche Diagnostics, Mannheim, Germany) confirmed the comparable expression of GFP-ALP proteins (data not shown). c.1559delT was already shown to be a recurrent mutation with no residual activity and relatively common in the Japanese ethnic background. These results indicate that both sequence variants are pathogenic mutations causing HPP.
3 DISCUSSION
Two siblings of HPP in our case showed considerable discordant severity in the fetal period. Although genotype-phenotype correlations have been widely described with regard to HPP (Zurutuza et al., 1999), several reports have demonstrated the discordance in calcification and fetal growth in HPP siblings with the same ALPL gene mutation (Macfarlane, Kroon, & van der Harten, 1992). Stevenson et al. (2008) showed discordant phenotype of HPP siblings, although both had compound heterozygous mutation in ALPL gene (c.526G>A and c.814C>T). Taketani et al. (2014) reported genotype of c.1559delT and c.T979C compound heterozygous mutations has a variance in clinical types including perinatal, infantile, and childhood type. The c.1559delT representing 41% of severe HPP alleles is a common mutation of the perinatal lethal form, especially in Japanese population (Michigami et al., 2005; Taketani et al., 2014; Watanabe et al., 2011). Similar to these previous reports, the present siblings with compound heterozygous mutations also had discordant clinical phenotype, and this discordance became remarkable on ultrasonography in the fetal period. For instance, sibling A presented the shortening of the long bones in the second trimester (−5 SD), while the shortening became evident in the third trimester (−3 SD) in sibling B. Additionally, shortening of the radius was evident at 34 weeks of gestation in sibling B, which was consistent with the cupping on postnatal X-ray. Therefore, the possibility of discordant phenotype even with the concordant genetic mutations of HPP should be taken into consideration during the follow-up using fetal ultrasonography.
It is demonstrated that recent introduction of enzyme replacement therapy using asfotase alpha could contribute to the improvement of clinical features: pulmonary and physical function as well as skeletal calcification in infants and young children (Whyte et al., 2012). In our case, the accurate diagnosis of HPP in sibling A as well as sequential follow-up of fetal ultrasonography, led us to early diagnosis of HPP in sibling B and prompt introduction of enzyme replacement therapy. Our experience emphasized the importance of the meticulous follow-up during the fetal period in siblings with HPP, which could enable early intervention to the affected infant.
ACKNOWLEDGMENTS
We thank all medical staffs in Keio University Hospital who contributed to the excellent patient care for this study.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.