Wieacker–Wolff syndrome with associated cleft palate in a female case
Abstract
Wieacker–Wolff syndrome is a rare congenital syndrome with few reported cases in the current literature. It is traditionally described in males as an X-linked recessive disorder associated with congenital contractures of the feet, progressive neurologic muscular atrophy, and intellectual delay caused by ZC4H2 mutations. The purpose of this paper is to present a female individual with a classic phenotype and cleft palate, a previously undescribed finding in this syndrome. Recent reports have demonstrated that females are rarely severely affected and phenotypic expression is difficult to predict [Zanzottera et al. (2017); American Journal of Medical Genetics Part A 173A: 1358–1363]. This case supports the unpredictability of Wieacker–Wolff syndrome severity and prompts future questions regarding female mutations and phenotypic expression.
1 INTRODUCTION
Wieacker–Wolff syndrome was first described by Peter Wieacker and Gerhard Wolff in 1985, in which they reported one family with six affected males (Wieacker, Wolff, Wienker, & Sauer, 1985). They described a syndrome of congenital contracture of the feet, progressive neurologic muscle atrophy, and associated intellectual disability that was inherited in an X-linked fashion. There were 22 females over three generations in the extended family and all had either mild phenotypic expression or were unaffected. Since its initial description, there are few published reports of Wieacker–Wolff syndrome, and it is current listed as an orphan disease (OMIM# 314580) with an estimated prevalence of <1/1,000,000 with only a total of approximately 30 cases described (ORPHA3454, ).
The gene for Wieacker–Wolff syndrome was first identified in 1987 and isolated to the pericentric region of the X chromosome, Xq11 (Kloos, Jakubiczka, Wienker, Wolff, & Wieacker, 1997). More recent studies have associated the Wieacker–Wolff phenotype with variants in the ZC4H2 gene (Hirata et al., 2013).
Mutations within the ZC4H2 gene have also been associated with Arthrogryposis Multiplex Congenita (AMC) with variable phenotypes (Hoff, Loane, Gilhus, Rasmussen, & Daltveit, 2011). Although the number of reported cases are limited, studies suggest that the phenotypic expression of ZC4H2 mutations is highly variable, particularly in females (Hirata et al., 2013, May et al., 2015, Zanzottera et al., 2017). One recent report identified five families, one of whom had a clinical diagnosis of Wieacker–Wolff syndrome, and three de novo cases with ZC4H2 mutations (Hirata et al., 2013). The report describes two affected female simplex cases, both of which were found to have deletions in the ZC4H2 gene. One individual had a more complex phenotype more consistent with Wieacker–Wolff syndrome (Hirata et al., 2013). Thus, until recently, heterozygous females were believed to only have a mild phenotypic expression, however these reports suggest that females can have a more severe phenotypic expression (Hirata et al., 2013, Zanzottera et al., 2017).
Variants within ZC4H2 mutations include a large number of features, however there are no prior reports of cleft palate among this population or specifically Wieacker–Wolff patients.
The purpose of this article is to present a female individual with Wieacker–Wolff syndrome who has a classic phenotype with a cleft palate.
2 CLINICAL CASE
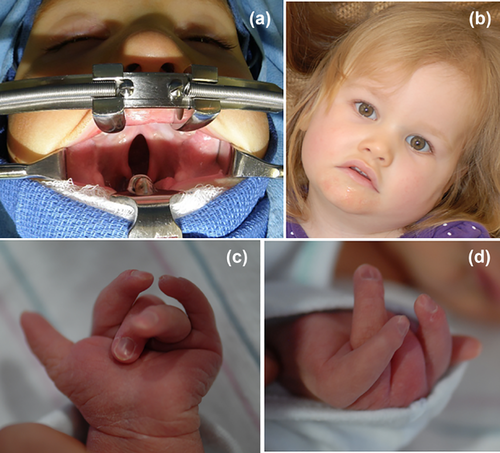
We present a 27-month-old female who was first evaluated shortly after birth for congenital contractures of her upper and lower extremities and a cleft palate. In addition, she was noted to have hypokinesia and poor feeding requiring gastric tube feeds. As she progressed in age, developmental delays became apparent.
The phenotypic presentation of the present individual includes: congenital contractures of the feet, distal motor weakness, radial and ulnar deviation of her digits, and intellectual disability. In addition, she has Pierre–Robin sequence with cleft palate and micrognathia. Her full list of characteristics is included in Table 1.
| Characteristic | Present case n = 1 | Simplex case 2, n = 1. Hirata et al. (2013) | Simplex case 3, n = 1 Hirata et al. (2013) | Family 3, n = 7 Hirata et al. (2013) | Family 4, n = 1 Hirata et al. (2013) | Family 5, n = 2 Hirata et al. (2013) | Simplex case 4 Zanzottera et al. (2017) | Family 6, n = 6 May et al. (2015) | Family 7, n = 4 May et al. (2015) | Family 8, n = 3 May et al. (2015) | Family 9, n = 4 May et al. (2015) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | c.148 C>T (p.Gln50*) | Deletion chrX: 63, 666, 909–64, 493, 512 | Deletion chrX 64, 049, 596–64, 370, 757 | c.601C>T (p.Pro201Ser) | c.637C>T (p.Arg213Trp) | c.637C>T (p.Arg213Trp) | Deletion chrX: 63, 898, 170–64, 327, 237 | c.197T>A (p.Leu66His) | c.637C>T (p.Arg213Trp) | c.225 + 5G>A (p.Val75in15aa) | c.53G>A (p.Arg18Lys) |
| Oculomotor apraxia | 1 | ||||||||||
| High arched palate | 1 | 1 | 2 | ||||||||
| Submucous cleft | 1 | ||||||||||
| Bifid uvula | 1 | ||||||||||
| Cleft palate | 1 | ||||||||||
| Micrognathia | 1 | 1 | 1 | ||||||||
| Narrow shoulders, thorax | 1 | 2 | 1 | 2 | |||||||
| Kyphosis, lordosis, scoliosis | 1 | 3 | 1 | 2 | |||||||
| Equinovarus feet, contracture of achilles tendon | 1 | 1 | 3 | ||||||||
| Radial deviation of fingers | 1 | 1 | |||||||||
| Ulnar deviation of fingers | 1 | 1 | 1 | ||||||||
| Wrist flexion contracture | 1 | ||||||||||
| Congenital hip flexion contractures | 1 | 1 | 1 | 1 | 1 | ||||||
| Congenital flexion contractures of elbows, knees | 3 | 1 | 6 | ||||||||
| Knee hyperextension | 1 | ||||||||||
| Distal muscle weakness | 1 | 1 | 1 | 3 | 1 | 1 | |||||
| Distal limb muscle atrophy | 1 | ||||||||||
| Retardation of motor development | 1 | 2 | |||||||||
| Intellectual disability | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 4 | 2 | 1 | |
| Dysarthria, deficit in expressive language | 1 | 1 | 1 | 1 |
- Table adapted from Hirata et al. (2013).
Initial genetic testing including microarray analysis and metabolic studies were non-diagnostic. Therefore, whole-exome sequencing was performed and identified a heterozygous de novo mutation, (c.148C>T [pGln50*]) of the ZC4H2 gene (transcript NM_018684) that resulted in a non-sense mutation. Additional X-inactivation studies were performed using a blood sample and showed a ratio of 74:26, which is consistent with a random pattern.
3 DISCUSSION
We present a female individual with a ZC2H4 mutation and classic phenotypic expression of Wieacker–Wolff syndrome and the first described with a cleft palate.
In comparing the present individual with other prior affected females, there are many similarities. The more severely affected females include Family 3 (Hirata et al., 2013), Simplex case 3 (Hirata et al., 2013), Family 6 (May et al., 2015), and Simplex case 4 (Zanzottera et al., 2017) who all have reported equinovarus, congenital contractures, distal motor weakness and intellectual disability. While none of these individuals have been reported to have a cleft in the hard palate, simplex case 3 was reported to have a submucous cleft and bifid uvula (Hirata et al., 2013).
In addition, a case report of a female with a contiguous gene deletion, which included the ZC4H2 and WTX gene was reviewed (Holman et al., 2013). This individual's genetic variations are associated with Osteopathia striata congenital with cranial sclerosis (OSCS). OSC's common presentation includes longitudinal striations of sclerotic bone along long axis of long bones, cranial sclerosis and high prevalence of cleft palate, and hearing loss. Given the prior lack of association of cleft palate with ZC4H2 mutation, the authors postulated that her cleft palate was likely related to the known association with WTX gene and not ZC4H2 deletion (Holman et al., 2013). However, this increases the support of a possible association between a ZC4H2 mutation and cleft palate. Figure 1.
Thus far, data on the ZC4H2 gene has focused primarily on zebrafish and neuromuscular, central neurologic systems and functioning (May et al., 2015). There is no available data that would suggest a mechanism for the cleft palate deformity.
The genetic mutations also differ amongst the severely affected females. Simplex cases 2, 3 (Hirata et al., 2013), and 4 (Zanzottera et al., 2017) have deletions within the ZC4H2 gene (2: ChrX: 63, 666, 909-64, 493, 512; 3: ChrX: 64, 049, 596-64, 370, 757, 4: chrX: 63, 898, 170–64, 327, 237), while Family 6 has a missense mutation c197T>A (p.Leu66His) (May et al., 2015). All previously reported cases with mutations in the ZC4H2 gene have either had missence variants or deletions; our individual represents the first reported case with a nonsense mutation.
Some of the phenotypic variations in these individuals have previously been postulated to be related to X-inactivation. Prior studies suggested that the mutated X is preferentially inactivated leading to a milder clinical picture/expression (Hoff et al., 2011). In our individual and the case reported by Zanzottera, X inactivation studies were consistent with a random pattern. However, these samples were from blood and lymphocytes respectively and thus may not represent all tissues. In addition, the nonsense variant could be a potential explanation for the more severe phenotype.
In conclusion, we present a female with Wieacker–Wolff syndrome with a classic phenotype and cleft palate. Her case supports the consideration to broaden the spectrum of associated phenotypes with this syndrome. Further research is still needed to better understand the clinical implications of the de novo mutations in Wieacker–Wolff syndrome.
ACKNOWLEDGMENTS
The authors thank their patient and her family for their ongoing participation in her clinical care at the University of Massachusetts and clinical photographs for this publication.