Adaptive and maladaptive functioning in Kleefstra syndrome compared to other rare genetic disorders with intellectual disabilities
Abstract
Detailed neurobehavioural profiles are of major value for specific clinical management, but have remained underexposed in the population with intellectual disabilities (ID). This was traditionally classified based on IQ level only. Rapid advances in genetics enable etiology based stratification in the majority of patients, which reduces clinical heterogeneity. This paper illustrates that specific profiles can be obtained for rare syndromes with ID. Our main aim was to study (mal)adaptive functioning in Kleefstra Syndrome (KS) by comparing and contrasting our findings to three other subgroups: Koolen-de Vries Syndrome, GATAD2B-related syndrome, and a mixed control group of individuals with ID. In total, we studied 58 individuals (28 males, 30 females) with ID; 24 were diagnosed with KS, 13 with Koolen-de Vries Syndrome, 6 with the GATAD2B-related syndrome, and 15 individuals with undefined neurodevelopmental disorders. All individuals were examined with a Vineland Adaptive Behavior Scale, mini PAS-ADD interview, and an Autism Diagnostic Observation Schedule to obtain measures of adaptive and maladaptive functioning. Each of the three distinctive genetic disorders showed its own specific profile of adaptive and maladaptive functioning, while being contrasted mutually. However, when data of the subgroups altogether are contrasted to the data of KS, such differences could not be demonstrated. Based on our findings, specific management recommendations were discussed for each of the three syndromes. It is strongly suggested to consider the genetic origin in individuals with congenital neurodevelopmental disorders for individual based psychiatric and behavioral management.
1 INTRODUCTION
Intellectual disabilities (ID) comprise a category of mental disorders with a broad clinical heterogeneity, including a high variation in neurobehavioral and neurocognitive functioning, physical impairments, and psychiatric comorbidity. The collective term ID is used for different conditions and is defined by several organizations. The American Association on Intellectual and Developmental Disabilities defines ID as a disability, which is characterized by significant limitations in both intellectual and adaptive functioning, which covers many daily social and practical skills and originates before the age of 18 (AAIDD, 2012). In previous editions, the Diagnostic and Statistical Manual of Mental Disorder (DSM) puts emphasis on classification based on IQ-level in addition to dysfunctioning in daily life. However, in its latest, fifth edition (DSM-5), the IQ-test scores are removed from the diagnostic criteria to reflect the importance of the overall ability of daily functioning (American Psychiatric Association, 2013). Both definitions do not take etiology into account. Consequently, clinical research as well as treatment procedures in the ID population are still based on general characteristics like IQ levels, resulting in huge clinical heterogeneity.
For clinical practice it is of great value to have specific knowledge about associated symptoms and the natural disease course of individual syndromes. Often, there are abnormalities in development and learning abilities as well as differences in the process of aging (Coppus, 2013; Nomura & Segawa, 2005). For more established syndromes with higher numbers of affected patients available, specific neurobehavioural, and cognitive profiles have been defined (Di Nuovo & Buono, 2011; Egger et al., 2013; van Rijn & Swaab, 2015; Wingbermühle et al., 2012). Consequently, these profiles should each to some extent have their own approach with respect to education, training, and (para)medical treatment.
With the advances in genetic techniques over the recent years, there are increasing numbers of ID cases in which a genetic cause can be identified. At this point, in the majority of the ID-population, a rare genetic variant can be identified that is causative for the ID (Gilissen et al., 2014; Vissers, Gilissen, & Veltman, 2016; Willemsen & Kleefstra, 2013). Little is known about neuropsychiatric aspects associated with these rare variants as the number of cases affected by a similar gene is mostly small thus making it difficult to conduct systematic studies in a homogeneous patient/syndrome group. Sometimes characteristics of behavior and neurocognitive functioning are described based on clinical observation. But even though the number of genes with rare variants in ID is extensive, this should not hold back the field in providing systematic clinical studies in single gene related syndromes as the number of patients who are diagnosed with the more recently novel defined syndromes, steeply increases.
This study focuses on Kleefstra Syndrome (KS). KS is a rare ID syndrome, caused by EHMT1 haploinsufficiency (Kleefstra et al., 2006). KS patients function in the range from a moderate to profound ID, rarely on a mild ID to borderline IQ (Bock et al., 2016; Samango-Sprouse et al., 2016). Several KS case reports mention autistic features, regression, and sleep problems (Schmidt, Nag, Hunn, Houge, & Hoxmark, 2016; Verhoeven, Egger, Vermeulen, van de Warrenburg, & Kleefstra, 2011; Verhoeven, Kleefstra, & Egger, 2010; Vermeulen et al., 2015). In addition, our clinical experience with these patients so far leads us to hypothesize that KS patients are more vulnerable to develop severe psychiatric disorders. To investigate this, we studied a large cohort of KS patients at adaptive functioning and clinical psychiatric domains.
In this study, contrast groups are used to make a comparison. Generally, control groups in medical research are composed on the basis of individuals wherein the studied condition is lacking, but who are otherwise with similar biological characteristics. For research in the area of intellectual disabilities (ID), this is often done by matching the controls on biological as well as developmental age (DiStefano et al., 2016). As the cause of the ID is not taken into account in this approach, control groups are largely heterogeneous. Such heterogeneous groups quickly differ from homogeneous (caused by similar genetic defect) syndrome groups. Therefore, we believe that a control group should consist of several homogeneous contrast groups, in which the genetic disorders are all well-defined.
At the start of the study, we selected two genetic syndromes to contrast our results to, based on our historical experience, the well-described monogenetic causes, biological age range, and level of functioning: Koolen-deVries Syndrome (KdVS) (Koolen et al., 2006; Sharp et al., 2006; Shaw-Smith et al., 2006) and GATAD2B related Syndrome (GS) (Willemsen et al., 2013). Systematic neurobehavioral research in these rare syndromes was not previously performed. KdVS is associated with a mild to moderate ID, rarely a more severe ID. Subsequently, problems in expressive language are often mentioned and in about half of the patients, there is a variety of behavioral problems, including features of autism, anxiety, psychosis, and ADHD (Koolen et al., 2015). A report on GS cases mentions severe ID and behavioral characteristics, like tics, hyperactivity, and sleep problems (Willemsen et al., 2013). Finally, a mixed group (MG) was composed of other rare (un)defined neurodevelopmental disorders. All together, the KdVS, GS, and MG groups form a cumulative control group (CC), which meets the more “traditional” format of contrasting a specific syndrome to a clinical heterogeneous control group.
To summarize, in this first report on adaptive and maladaptive behavioral functioning in KS, we systematically contrast monogenetic syndromes to identify syndrome specific profiles. We hypothesize that KS patients are more vulnerable to develop severe psychiatric disorders, especially autism spectrum disorders, mood disorders, and psychosis. Our second hypothesis is that results emerge more clearly, when the data of KS patients are contrasted to the data of patients with other well-defined genetic syndromes.
2 MATERIALS AND METHODS
2.1 Participants
Included were 58 patients (28 males, 30 females) subdivided into four groups: Kleefstra Syndrome (KS, n = 24), Koolen-de Vries Syndrome (KdVS, n = 13), GATAD2B syndrome (GS, n = 6), and a mixed group (MG, n = 15). The participants in the KS, KdVS, and GS group cover all the identified subjects with these syndromes in the Netherlands and Belgium. Due to rarity of individual genetic syndromes this number was maximum to achieve. In addition, the KdVS, GS, and MG-groups are taken together for statistical analysis and then referred to as cumulated control group (CC) (Figure 1). Patient characteristics are summarized in Table 1. Patients with KS, KdVS, and GS were invited to participate from the department of Human Genetics, Radboud University Medical Centre, the Netherlands. The MG subjects have very rare genetic variants (Table 2) or an undefined condition underlying their neurodevelopmental disorder and are recruited both from the department of Human Genetics(HG), Radboud university medical center, the Netherlands, and from the department of Child- and Adolescent Psychiatry (CP), Karakter Horst, the Netherlands. Informed consent was obtained by legal representatives and included in the participant file. The regional medical ethical committee (medical research ethics committee CMO/METC Arnhem-Nijmegen, the Netherlands) approved the study (NL43187.091.13), which was performed in full accordance with the Declaration of Helsinki.

| Groups | Subgroups | N | Genetic specification (N) | Male:Female (N) | Biological age range (min–max in years) | Biological age Mean ± SD |
|---|---|---|---|---|---|---|
| Kleefstra syndrome (KS) | 24 | EHMT1 gene mutation (n = 8)/microdeletion (n = 16) | 9:15 | 3–37 | 15.42 ± 10.42 | |
| Cumulated control group (CC) | 34 | 19:15 | 3–40 | 14.29 ± 10.13 | ||
| Koolen-de Vries syndrome (KdVS) | 13 | KANSL gene mutation (n = 1)/microdeletion (n = 12) | 6:7 | 5–34 | 18.31 ± 10.70 | |
| GATAD2B-related syndrome (GS) | 6 | GATAD2B gene microdeletion (n = 6) | 2:4 | 3–40 | 16.50 ± 13.64 | |
| Mixed group (MG) | 15 | a | 11:4 | 3–30 | 9.93 ± 6.33 | |
| Total | 58 | 28:30 | 3–40 | 14.76 ± 10.18 |
| aGenetic variants identified in the mixed group (MG). | |
| Genetic variant | N |
| ANKRD11 mutation | 3 |
| SIN3A mutation | 3 |
| PACS1 mutation | 2 |
| FOXP2 mutation | 1 |
| 2p16.3 microdeletion | 1 |
| FBOX17 gene | |
| 7q11.22 microdeletion | 1 |
| AUTS2 gene | |
| 7q36.1 microdeletion | 1 |
| 17p13.3 microduplication | 1 |
| YWHAE gene | |
| Unknown result | 1 |
| Refused the advice to perform whole exome sequencing | 1 |
| Total | 15 |
| Autism spectrum disordera | Major depressive disorderb | Anxiety disorderb | (Hypo)maniab | Obsessive compulsive disorderb | Psychosisb | Onspecified disorderb | Regressionc | Sleep problemsc | |
|---|---|---|---|---|---|---|---|---|---|
| Kleefstra syndrome (KS, n = 24) | 22/23 (95.7%)* | ||||||||
| Present (%) | 4/24 (16.6%)* | 6/24(25%) | 3/24(12.5%) | 4/24 (16.6%)* | 5/24 (20.8%)* | 2/24 (8.3%)* | 4/24 (16.6%) | 10/24 (41.6%) | |
| Past (%) | 10/24(41.6%)* | 11/24(45.8%) | 7/24(29.2%) | 8/24 (33.3%)* | 7/24 (29.2%)* | 9/24 (37.5%)* | 12/24 (50%) | 19/24 (79.2%) | |
| Cumulative control group (CC, n = 34) | 17/34 (50%) | ||||||||
| Present (%) | 0/27 (0%) | 7/27 (26%) | 4/27 (15%) | 2/27 (7.5%) | 1/27 (4%) | 0/27 (0%) | 1/27 (4%) | 15/27 (56%) | |
| Past (%) | 6/27 (22%) | 14/27 (52%) | 7/27 (26%) | 3/27 (11%) | 1/27 (4%) | 4/27 (15%) | 8/27 (30%) | 23/27 (85%) | |
| KoolendeVries syndrome (KdVS, n = 13) | 7/13 (53.8%) | ||||||||
| Present (%) | 0/13 (0%) | 4/13 (30.8%) | 2/13 (15.3%) | 0/13 (0%)* | 1/13 (7.7%) | 0/13 (0%) | 1/13(7.7%) | 7/13 (53.8%) | |
| Past (%) | 1/13 (7.7%) | 9/13 (69.2%) | 4/13 (30.8%) | 1/13 (7.7%)* | 1/13 (7.7%) | 1/13 (7.7%) | 4/13 (30.8%) | 9/13 (69.2%) | |
| GATAD2B syndrome (GS, n = 6) | 1/6 (16.5%)* | ||||||||
| Present (%) | 0/6 (0%) | 2/6 (33.3%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 3/6 (50%) | |
| Past (%) | 3/6 (50%) | 3/6 (50%) | 1/6 (16.7%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 4/6 (66.7%) | 6/6 (100%) | |
| Mixed control group (MG, n = 15) | 9/15 (60%) | ||||||||
| Present (%) | 0/8 (0%) | 1/8 (12.5%) | 2/8 (25%) | 2/8 (25%) | 0/8 (0%) | 0/8 (0%) | 0/8 (0%) | 5/8 (62.5%) | |
| Past (%) | 2/8 (25%) | 2/8 (25%) | 2/8 (25%) | 2/8 (25%) | 0/8 (0%) | 3/8 (37.5%) | 0/8 (0%) | 8/8 (100%) | |
| Total (n = 58) | 39/57 (68.5%) | ||||||||
| Present (%) | 4/51 (7.8%) | 13/51 (25.5%) | 7/51 (13.7%) | 6/51 (11.8%) | 6/51 (11.8%) | 2/51 (3.9%) | 5/51 (9.8%) | 25/51 (49%) | |
| Past (%) | 16/51 (31.3%) | 25/51 (49%) | 14/51 (27.4%) | 11/51 | 8/51 (15.7%) | 13/51 (25.5%) | 20/51 (39.2%) | 42/51 (82.4%) |
- a Measured with the ADOS and presented as a life time prevalence, because ASD is a developmental disorder. A diagnosis was made, based on the comparison scores on the ADOS-2 with a cut-off of 5 and above. In the KS group, 1 of the participants was not able to complete the ADOS-2 module 1, due to aggression.
- b Measured with the mini PAS-ADD.
- c symptom scales: Regression is measured in subdomain A of the mini PAS-ADD. A score of ≥2 is indicative for loss of functions (minimum score = 0;maximum score = 6) and is seen as regression in this cohort. Sleep problems are measured in the subdomains D (stopped sleeping in the night), E (problems falling a sleep) and F (early awaking <1 hr to normal or disturbed sleep in the night; question 11 and 12 of section F). A score of ≥1 is indicative for at least one of these. The scores are corrected for anhedonia (question 13), which is also measured in section F.
- * Significant different prevalence (p < 0.05).
2.2 Instruments
In this study, we aimed to focus solely on clinical parameters, obtained through clinical interviews (VABS, mini PAS-ADD), or by direct testing of the subject (ADOS-2), in order to obtain objective measurements.
2.2.1 Vineland adaptive behavior scale (VABS)
The Dutch adaptation of the Vineland Adaptive Behavior Scale (Sparrow, Balla, & Cicchetti, 1984) is a widely used clinical interview, which determines the level of adaptive functioning of people with an intellectual disability. The VABS consists of three domains: communication skills, daily living skills, and social skills. This instrument has a good reliability and validity in this specific population (de Bildt, Kraijer, Sytema, & Minderaa, 2005). Primary caregivers were interviewed about the participants.
2.2.2 Autism diagnostic observation schedule (ADOS)
ADOS is a semi-structured play to assess autism features (Lord et al., 1989; Lord, Rutter, DiLavore, & Risi, 1999). It is performed by a certified psychologist or psychiatrist (in this cohort, the first author, K.V.) and consists of four modules, based on the (developmental) age and language capacity of the participant. Module 1 is preverbal to minimal verbal capacity, module 2 is used by the capacity to use short sentences, and module 3 is used by a normal verbal capacity, but still includes play elements. Module 4 can be used in normal functioning adults. In this studies module 1, 2, and 3 are performed. For each of these modules is a comparison score available, which corrects for biological age and language capacity. The cut-off for clinical suspicion of an autism spectrum disorder is from 5 and above (moderate to severe suspicion).
2.2.3 The mini psychiatric assessment schedules for adults with developmental disabilities (mini PAS-ADD)
The mini PAS-ADD (Moss, Costello, Simpson, & Patel, 1997); in Dutch translation (Janssen & Maes, 2012); is used to determine behavioral problems and psychiatric disease in subjects with an intellectual disability by interviewing the proxy. It consists of 86 items on a 4-point scale: 0 (symptom not present)—3 (symptom is severe). The interview is divided into seven subscales: Depression, Anxiety, Obsessive/Compulsive disorder, Hypomania/Mania, Psychosis, Unspecified disorder, and Autism. All criteria are based on the International Classification of Diseases (ICD-10). This instrument has proven psychometric qualities in this specific ID adult population, but not yet in a population sample with children (Janssen & Maes, 2012; Prosser et al., 1998). To our opinion, however, we believe that it is also applicable to adolescents/ children with ID, because many adjusted criteria for people with ID overlap with the criteria for children. For example, an irritable mood is more often seen in children as well as people with ID as an expression of a depressive disorder.
The mini PAS-ADD was completed for all participants, except the ones who were referred from the CP outpatient clinic. They had a regular anamnestic interview at intake.
2.3 Procedure
All participants were visited at home, except seven cases from the control group who were referred by the Child Psychiatric department. Those were examined at the outpatient clinic for Intellectual Disabilities & Child Psychiatry (Horst, the Netherlands) as a part of a normal diagnostic procedure.
All home visits and intakes were performed by the same investigator (K.V.) in the presence of a research assistant.
The home visit started by interviewing the parents with the VABS and the mini PAS-ADD. The participant was mostly present in the same room and was able to get used to the presence of the investigator. After this, one of the modules of the ADOS-2 was chosen and made ready in a quiet and familiar room of the house (living room, kitchen). During the ADOS-2 one of the parents was present in the same or an open adjacent room, filling in questionnaires. The ADOS-2 was videotaped and scored conform the manual of the ADOS-2.
For the controls, who were already seen for clinical intake, the VABS, and the ADOS were performed after a normal intake procedure, including history taking, and observation. These tests were performed in the same room setting as the intake and (one of) the parents were also present, filling in questionnaires. Because of time and capacity of the participants, the mini PAS-ADD was not carried out.
2.4 Statistical analyses
Analysis were performed using SPSS 22.
2.4.1 Adaptive functioning
The scores of the VABS subdomains were first plotted against normative data (decile scores) and subsequently standardized (into Z-scores). In addition, a covariate analysis was performed to test the influence of biological age.
2.4.2 Maladaptive functioning
For the means of prevalence rates, the cut-off scores were used conform the manuals of the ADOS and mini PAS-ADD. A Fisher's exact test was performed to compare point prevalences (current episodes) as well as lifetime prevalences between the several groups. Scores of each syndrome (n > 5) were first plotted against all other participants to systematically test for syndrome specific psychopathology, followed by a Fisher's exact test between the several subgroups.
After this, the ADOS comparison scores and subscales scores on the mini PAS-ADD were also compared to complete tendencies within the clinical picture. For this purpose, non parametric tests for independent samples were used; the Mann–Whitney test for comparing KS against CC, a Kolmogorov–Smirnov for the smaller samples of as well KdVS as well as GS against all other participants. Finally, the Kruskal–Wallis test to screen for overall tendencies between the several contrast groups.
3 RESULTS
3.1 Adaptive functioning
The first step was to calculate and contrast adaptive functioning in KS and CC, based on the deciles for the Dutch population of people with ID (decile scores, Figure 2). The adaptive functioning of the participants was slightly lower compared to the mean of the total ID population in the Netherlands (Figure 2a, KS vs. CC, a decile score of five reflects the mean of the total Dutch ID population). There is a clear difference in level of adaptive functioning with CC subjects performing better at all domains in comparison to KS.
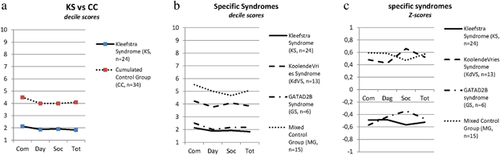
Because of the large variability within the CC group, a statistical mean was calculated. The scores of the several groups were also separated from each other and again presented as deciles (Figure 2b: KS, KdVS, GS, and MG). This shows more specific profiles for each of the syndromes. However, in the calculation of the Dutch decile scores the patients with severe to profound ID are slightly underrepresented. So to draw clear conclusions, also for the syndromes with lower levels of adaptive functioning, we contrasted them to each other by calculating Z-scores. This resulted in even more specific profiles for each of the syndromes and these results are displayed in Figure 2c.
Finally, an analysis of the VABS scores with biological age as a covariate was performed to assess this component in the different groups. We expected a positive correlation between the biological age and the developmental age in the age category of our patients (3–40 years, Table 1). A significant result was only seen in the CC group and the MG subgroup (Kendall's tau,correlation coefficient in CC = 0,272, p = 0,027, and in MG = 0,518, p = 0,009). This means that in the syndrome groups (KS, KdVS, and GS), no linear association was demonstrated between biological age and the level of adaptive functioning.
3.2 Maladaptive functioning
After assessment of adaptive functioning, maladaptive functioning was determined based on the ADOS and mini PAS-ADD scores. Prevalence rates for major psychiatric disorders and symptoms are shown in Table 2 for each of the groups.
To measure whether these prevalence rates are significantly different for each of the syndromes, several analyses were performed. Initially, KS and CC were contrasted against each other at several domains of the mini PAS-ADD (measuring episodic psychopathology) and the ADOS (comparison) scores. Prevalence rates were tested with a Fishers exact test and symptom scores with a Mann–Witney test.
The results are displayed in Tables 2 and3. Significant higher prevalences of autism spectrum disorders (p = 0.001), current major depressive disorder (p = 0.043), and current OCD (p = 0.033) were demonstrated in KS. The prevalence of psychosis was not significantly higher (p = 0.066 for past episode), but a Mann–Whitney test of the psychotic subscale showed significantly severe symptom scores for KS as well for current psychosis (p = 0.015) as well as in the past (p = 0.005). Most strikingly, all KS-participants with a biological age above 15 years have (had) a psychosis.
| Group (n) | ADOS2 comparison score range (min–max) | ADOS2 comparison score mean ± SD | Clinical diagnosisa (% of patients) |
|---|---|---|---|
| Kleefstra syndrome (n = 23)b | 42–10 | 6.74 ± 1.60 | 22/23 (95.7%)c |
| Cumulated control group (n = 34) | 1–10 | 4.76 ± 2.38 | 17/34 (50%) |
| KoolendeVries Syndrome (n = 13) | 1–10 | 5.08 ± 2.53 | 7/13 (53.8%) |
| GATAD2B mutation (n = 6) | 1–8 | 3.17 ± 2.58 | 1/6 (16.7%) |
| Mixed Group (n = 15) | 1–8 | 5.13 ± 2.07 | 9/15 (60%) |
| Total (n = 58) | 1–10 | 5.56 ± 2.30 | 39/58 (67.2%) |
- a Clinical diagnosis is based on a ADOSscore of 5 and above together with an expert opinion.
- b One drop-out because of severe aggression during ADOS.
- c The participant with a comparison score of 4 was severely sedated and in the isolation room.
Secondly, results of the KdVS group were compared against all other participants, using a Fisher's exact test for prevalence rates and a Kolmogorov–Smirnov test for the severity of symptoms. This resulted in significantly lower prevalence of life time depression (p = 0.041) and current OCD (p = 0.048). Severe levels of anxiety symptoms in the past (p = 0.022) were demonstrated KdVS. For GS, exact the same procedure was followed, resulting in a significantly lower prevalence of ASD (p = 0.005). After this, the same was done for MG, but no significant results were demonstrated for prevalence rates neither for symptom scores. This confirms our second hypothesis that mixing blurs the outcome.
Subsequently, all separate groups(KS, KdVS, GS, and MG) were contrasted against each other. Prevalence rates were again compared by Fisher's exact tests and resulted in discriminating prevalences for ASD (p = 0.00) and current OCD (p = 0.042). An overall Kruskal–Wallis test for symptom scores showed a significant differences at the ADOS comparison scale (p = 0,01) and at the psychotic symptoms (past) scale (p = 0,033).
Our results enabled differentiation between the syndromes, which is visualized for psychiatric comorbidity in Figure 3 and further outlined hereafter.
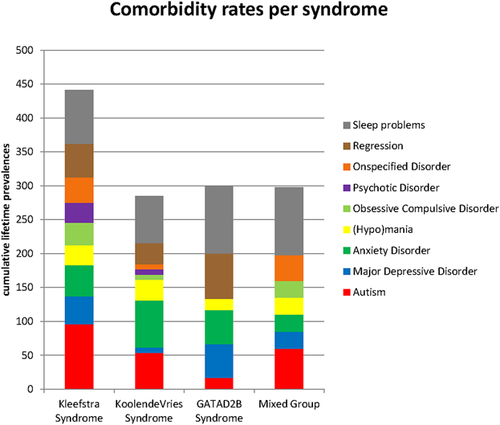
3.2.1 Kleefstra syndrome (KS)
Participants with KS have a low level of adaptive functioning with a fairly uniform pattern of functioning, except for socialization skills. This is in line with the autism scores, measured with the ADOS. All KS participants fit the diagnostic criteria for ASD and there is a significant difference in the prevalence of ASD in contrast to the control group as well as to the specific syndromes. KS-participants also show significant higher prevalence of current depressive episodes and OCD. Symptom scores for OCD (present and past) and psychosis (past) are significantly severe, resulting in a discriminating clinical picture: all KS subjects in this cohort suffer from ASD, are vulnerable to severe forms of OCD, depression, and psychosis. In addition to this, 100% of the patients above the age of 18 show a decline in functioning, which was not reversible. This was proceeded by severe sleep problems. The decline in functioning results in the absence of a linear relationship between biological age and adaptive functioning. Our hypothesis is that this regression is due to suffering from an (unrecognized) psychotic episode. The prevalence of psychosis is about 10 times higher compared to the general ID population, where the prevalence is around 3% (2014) and also significantly higher compared to the contrast groups, with a prevalence of 4% in CC. It is important to highlight that none of the participants received an optimal treatment for this. So conclusively, we confirmed our first hypothesis: KS patients are extremely vulnerable to develop severe psychiatric disorders and should be carefully monitored for this.
3.2.2 Koolen-deVries syndrome (KdVS)
Patients with KdVS have a moderate level of functioning compared to the general population with an ID in the Netherlands. Obvious is the strength on socialization in the patients without autism (6/13). This was also observed by clinical observation. The KdVS cohort showed low prevalence of depressive disorders as well as OCD. The subscale scores of present anxiety symptoms were more severe, but not resulting in a higher prevalence of anxiety disorder. Based on clinical observation there is a suspicion of AD(H)D in the participants with this syndrome; they had a surplus of defocused attention, which made it difficult to focus attention and complete tasks.
3.2.3 GATAD2B-related syndrome (GS)
Patients with GS have low levels of adaptive functioning with a strength on social functioning and weaknesses in communication skills. Problems with expressive language skills are evident, whereas, the non verbal communication is comparable to their overall level of functioning. This deficit in verbal capacity is sometimes mistaken as a symptom of autism. However, our cohort shows that ASD occurs significantly less often in GS. Sleep problems, mood and anxiety disorders are common in this syndrome, however, these prevalence rates were not significantly discriminating in this small group. Regression was also reported in 2/3 of the group and was temporary. However, in some cases this regression stopped after psychiatric diagnosis and treatment.
3.2.4 Mixed control group (MG)
The level of adaptive functioning in MG subjects was averaged and compared to the total population with an ID. Statistic analysis did not show differences in any of the domains. This confirms our hypothesis that mixing genetic subgroups blurs the clinical picture.
The prevalence of ASD is comparable to prevalence rates mentioned in literature for people with an ID (2014). The prevalence rates for the remaining categories can be overshadowed by the bisection of the participants, because half of them completed the mini PAS-ADD interview. The prevalence of psychosis deviates from the literature, even when taken into account that the prevalence for psychosis is already higher in patients with ID.
Following our results, we propose clinical guidelines for management of psychiatric comorbidity of KS, KdVS, and GS as summarized in Table 4. These guidelines are based on current clinical guidelines, enhanced with our specific experiences.
| Syndrome | Target symptoms to be especially aware of in diagnostic procedures | General guidelines for treatment of the target symptoma | Medication advise for severe casesb |
|---|---|---|---|
| KS | Autism | Reduce stimuli | Low dosage of antipsychotics to reduce hypersensitivity. |
| Antidepressive agents to reduce severe obsessive compulsive symptoms | |||
| Psychosis &Regression (preceded by sleep problems) | Reduce stress, restore a normal sleep pattern (day–night rhythm) and immediate treatment with medication. | Normal to high dosages of atypical antipsychotics (preference for Olanzapine to restore sleep or Aripiprazole) | |
| Depressive Mood disorder | Activation and in severe cases antidepressive medication. | Antidepressive agents conform clinical guidelines for depressive episodes. | |
| KdVS | Anxiety | Psychotherapeutic treatment procedures, including psychomotor therapy (social anxiety), EMDR (specific traumata) and musical therapy (generalized anxiety). | Not observed. Procedures conform clinical guidelines for anxiety disorders is advised . |
| ADHD | Daily structure and routine. Reduction of stimuli during daytime. | Stimulant agents conform clinical guidelines for ADHD. | |
| GS | Problems with expressive language | Speech therapy to optimize non-verbal skills. | None |
| Sleep problems | Day night rhythm, routine going to bed. Reduction of stimuli during daytime; including rest moments. | Low dosage of antipsychotics to reduce perceptual hypersensitivity. | |
| Anxiety and Mood disorders | Activation and psychotherapeutic intervention, including psychomotor therapy (social anxiety), EMDR (specific traumata) and musical therapy (generalized anxiety). | Antidepressive agents conform clinical guidelines. | |
| Regression | Psychiatric consultation for further diagnostics. | Dependent on underlying disorder. |
- a Observed, advised and effective in our patient population.
- b Observed, advised and effective in our patient population, always together with psychiatric consultation.
4 DISCUSSION
In this paper, we provide an overview of the profiles of adaptive and maladaptive functioning of the largest KS cohort studied so far, showing that these are markedly different from several other rare genetic disorders with ID.
KS patients showed low levels of functioning and an abnormal course, characterized by sudden regression of functioning during adolescence. Adult patients suffered without exception from severe and persistent regression. Interestingly, all patients who suffered from regression showed high symptom scores of psychosis, but this was not recognized in most of them and none of the patients received optimal antipsychotic treatment. In addition to this, there is a high prevalence of psychosis in KS. We hypothesize that the sudden and persistent regression is an expression of a psychotic episode and needs to be treated likewise. In our KS-cohort, optimal treatment with antipsychotic drugs was achieved at normal to high dosages of atypical antipsychotic drugs.
Besides, an extremely high prevalence of ASD was found in KS as well as an extra vulnerability to develop OCD and increased levels of depressive episodes. The question arises whether the depressive symptoms can be attributed to a comorbid mood disorder or are an expression of a psychosis as well (also known as “negative” symptoms). Disentangling this can be complicated in patients with low levels of adaptive functioning. It can be questioned whether the observed OCD symptoms are related to an OCD sensu strictu or (at least partially) to the diagnosis of autism (stereotypies and restricted behaviors), depression, and/or psychosis.
The analyses based on contrast groups enabled us to discuss associated features for both KdVS as well as GS. Participants with KdVS showed low rates of associated psychopathology. They sometimes experience (severe) symptoms of anxiety, although this is usually not leading to a formal diagnosis of anxiety disorder. Perhaps, these symptoms might be a result of underlying problems with executive functioning. Further neuropsychological testing is recommended for this. They may also be related to their level of adaptive functioning, since anxiety is a common phenomenon in infants/preschoolers.
Participants with GS in our sample were more vulnerable to mood and anxiety symptoms, when contrasting their profiles to those of the other disorders. However, this should be interpreted with caution, since the GS cohort represents only six participants and analysis of additional patients is required to confirm this observation.
Interestingly, the adaptive functioning of the CC group does not provide insight into the strengths and weaknesses of the different gene-related subgroups. This may imply that it may be more fruitful to classify the ID population into categories based on underlying genetic defect instead of IQ level.
Additionally, the developmental pattern of adaptive functioning also varied between the different syndromes. This suggests that during life the level of adaptive functioning should be assessed periodically and in relation to maladaptive functioning. In the three syndromes, there was no linear relationship between aging and the level of adaptive functioning. Regression toward lower levels of functioning commonly occurs as well as stagnation of learning abilities. This regression may be due to lack of diagnosis and treatment of major psychiatric illness in people with ID, a phenomena that has been described for patients with mild intellectual disability (Kok, van der Waa, Klip, & Staal, 2016). It is important for (health) care, social goals, and school functioning to figure out how these learning curves run for each of the syndromes. We suspect that prevention of over- and under stimulation results in lower rates of (secondary) psychopathology, less stress, a higher quality of life, and also less health care costs.
The prevalence of psychopathology in the total group matches the prevalence of psychopathology in ID reported previously in literature (Borthwick-Duffy, 1994; Cooper, Smiley, Morrison, Williamson, & Allan, 2007; Dykens, 2000; Horovitz et al., 2011; Tsakanikos & McCarthy, 2014). Nevertheless, each syndrome shows its own pattern of adaptive as well as maladaptive functioning. We suggest specific target symptoms for diagnostic and treatment procedures for each syndrome in Table 3. The ADOS is discriminating in assessing autism features, even in the syndromes with the lowest levels of adaptive functioning and preverbal capacities. The ability of using comparison scores between the specific modules, fits the need for personalized diagnostics within a research design. It is not always regarded as valuable to diagnose ASD in moderate to profound ID, because the approach and guidance for these patients already include some characteristics of the ASD methods: visualization with pictograms and a lot of daily routine for example. However, the approach and guidance are essentially different in ID with and without ASD regarding socialisation (ASD patients need more stimulation and training in this) and limiting perseveration and stereotype interests and behavior. And last but not least, the range of comorbid psychopathology differs between these groups, further emphasizing syndrome specific guidance.
The strength of this study is the detailed measuring of psychopathology in the largest cohort of KS patients so far, and contrasting these to profiles of other rare genetic disorders with ID. Psychopathology and consequent maladaptive functioning have a direct impact on adaptive functioning. Knowledge about associated psychopathology adds a specific focus to the existing literature about these genetic syndromes. In general, behavioral problems are rarely specified in literature on genetic syndromes with ID, although they are one of the major problems for parents and caregivers to deal with in daily life. On the other hand, studies on psychopathology in ID focus on IQ-related groups, which are clinically heterogeneous. Stratification based on the genetic origin provides specific profiles for adaptive as well as maladaptive functioning compared to the “old fashioned” classification by IQ level. This is urgently needed to personalize the treatment of psychopathology as well as the counseling for people with ID.
One of the restrictions of this and any similar study on rare syndromes is that the statistical power is limited. Despite this, we were able to show significant differences at several domains, using proper statistical test. As such, the differences in neurocognitive profiles are not likely attributed to false positive results. Secondly, it remains difficult to use proper diagnostic procedures, which fit both the special needs of the total ID population as well as the specific individual symptoms and specific syndromes. However, the ID population pre-eminently needs personalized diagnostic procedures. A last limitation is the composition of the subscale (hypo)mania within the mini PAS-ADD, which includes several features overlapping the DSM-V classification of ADHD. We recommend an additional instrument for measuring ADHD (symptoms) in future studies. Furthermore, we recommend the use of the ADOS to discriminate autism features, even in the extremes of adaptive functioning. Future studies should include adapted neuropsychological tests in order to obtain a detailed neurocognitive profile for each of the genetic syndromes, which relate cognitive measures to adaptive as well as maladaptive functioning. Other recommendations for future research include follow-up of the participants over several years to examine the natural course of adaptive and maladaptive functioning and anticipate on proper intervention in case of evolving psychopathology.
ACKNOWLEDGMENTS
We thank the patients and their families for participation. The study arose from collaboration of the departments of Human Genetics and Psychiatry of the Radboud University Medical Center, Karakter child and adolescent psychiatry, and the Vincent van Gogh Centre of Excellence for Neuropsychiatry, Venray.