Achondroplasia: Really rhizomelic?
Abstract
Achondroplasia is the most common form of short limb dwarfism in humans. The shortening of the limb lengths in achondroplasia is widely described as “rhizomelic.” While this appearance may be convincing clinically, the description is not necessarily true or helpful radiologically. The aims of this study, were therefore, to determine whether rhizomelic shortening is a true feature of achondroplasia at diagnosis in infancy. Humeral, radial, femoral, and tibial diaphyseal lengths were recorded by two independent observers from 22 skeletal surveys of infants with achondroplasia and compared with 150 normal age-matched control subjects. Upper and lower limb bone length ratios (radial/humeral and tibial/femoral lengths, respectively) in both groups were compared using an unpaired t-test. Mean upper limb length ratios were statistically higher within the achondroplasia group at 0.87 ± 0.04 (n = 22, mean age 70 ± 94 days) compared to normal controls at 0.79 ± 0.02 (n = 150, mean age 113 days ± 88 days; P < 0.0001). Lower limb length ratios were not significantly different between groups (0.84 ± 0.04 vs. 0.83 ± 0.02, P = 0.46). There was good inter-observer agreement of limb length measurements, with an average measurement difference of 0.1 ± 1.4 mm. In conclusion, infants with achondroplasia demonstrate statistically significant rhizomelic shortening within the upper limbs, but not lower limbs at diagnosis, compared to normal controls. The term “rhizomelic shortening” in relation to achondroplasia should be reserved when describing upper limb proportions. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Achondroplasia is the most common form of short limb dwarfism in humans with an incidence of between one in 10,000–300,000 live births [Horton et al., 2007]. In over 80%, it is caused by a spontaneous point mutation in the fibroblast growth factor receptor 3 (FGFR 3) gene [Yamanaka et al., 2003; Horton et al., 2007] leading to abnormal regulation of chondrogenesis at the growth plate, and reduction in longitudinal bone length [Wright and Irving, 2012; Qi et al., 2014].
The shortening of the limb lengths in achondroplasia is widely described as “rhizomelic” [National Organization for Rare Disorders, 2002; Castriota-Scanderbeg and Dallapiccola, 2005; Grunebaum, 2005; Lovell et al., 2005; Richette et al., 2008; Laederich and Horton, 2010]. This term implies that proximal segments of the limbs are disproportionally shorter than distal segments [Vanhoenacker et al., 2001]. While this appearance may be convincing clinically, possibly due to differences in positioning of skin creases on the patient, the description is not necessarily true, or helpful radiologically. The basis for the terminology is likely to have stemmed from descriptions within numerous case reports published during the 1900's [Milne, 1910; Pritchard, 1911; Pritchard, 1928; Morgan, 1930] whereby, radiological assessment of skeletal dysplasias was not routinely employed, and was still in its infancy.
Nevertheless, publications on the topic have been conflicting. In 1967, Langer et al. [1967] published qualitative and quantitative findings of radiographic manifestations in achondroplasia. The authors reported a subjective rhizomelic pattern of shortening in the upper limbs, but absent or only very mild rhizomelic shortening in the lower limbs when compared to published normal standards [Maresh, 1955].
Further research conducted in 1975 by Nehme et al. [1976], concluded that lower limb rhizomelic shortening was a feature of achondroplasia; as expected average femoral lengths were a greater number of standard deviations shorter than tibial lengths, when compared with published normal standards [Anderson and Green, 1948].
To our knowledge, there are no published studies that formally assess both the radiographic measurements of upper and lower limb proportions in achondroplasia with comparison to a locally derived normal (or average statured) population. We hope that by clarifying the type of shortening present, we can improve the accuracy of the radiographic descriptions of the condition.
The objective of this study, was therefore, to determine whether rhizomelic shortening is a genuine radiographic feature of infants with achondroplasia at the point of diagnosis.
MATERIALS AND METHODS
We retrospectively searched our clinical radiological database over a 10 year period for patient reports containing the words “achondroplasia,” “achondroplastic,” and “achondroplast.”
Achondroplasia Patient Group
The diagnosis of achondroplasia was confirmed by the presence of typical skeletal radiology and clinical findings for each case. Measurements of limb proportions were not used to confirm the diagnosis. All cases were reported between January 2005 and August 2014, and included patients who were being treated locally and also those referred from other institutions for local review.
Skeletal surveys or “babygrams” (full body radiographs of a neonate) specifically obtained for the diagnosis of a skeletal dysplasia, were retrospectively reviewed. The maximal diaphyseal bone lengths for the humerus, radius, femur, and tibia using the tools of the workstation on our local Picture Archiving and Communication System software (GE Centricity RIS- i5.0). The laterality of the limb measured was also recorded as not all skeletal surveys obtained for a skeletal dysplasia included both right and left limbs.
Control Subject Selection
Post-mortem skeletal survey imaging performed at our institution between June 2011 and August 2014 were chosen to create an average statured (i.e., “normal”) control group, as imaging of each patient would include both upper and lower limbs (rarely conducted in healthy live patients due to the increased radiation burden). Each post-mortem skeletal survey included up to 20 images of the axial and appendicular skeleton in line with current Royal College of Radiologists and Royal College of Paediatrics and Child Health guidelines [Standards for Radiological Investigations of Suspected Non-Accidental Injury, 2008]. Radiographs were all acquired at our institution on a Ysio digital imaging system with wireless detector (Siemens AG Medical Solutions, Erlangen, Germany).
Cases were age-matched where possible, with skeletal surveys excluded in any child aged above 1 years of age, with a known history of growth retardation, family history of bone dysplasia, or premature delivery. All skeletal surveys included had been reported by two consultant paediatric radiologists as part of our routine clinical practice. None of the patients in the control group were identified to have bone abnormalities in the limbs at the time of reporting, or at autopsy.
Bilateral maximal diaphyseal bone lengths for upper and lower limbs were measured using tools of the PACS workstation. Average limb ratios in the control group were calculated from the mean measurements between right and left limb measurements.
Data Analysis and Measurements
Upper and lower limb length ratios were calculated for each subject. Upper limb length ratio was defined as the radial length divided by the humeral length. Lower limb length ratio was defined as the tibial length divided by the femoral length.
To assess inter-observer variability, all measurements in the achondroplasia group were independently repeated by a second observer (AC). The second measurement was obtained to verify limb lengths and prevent any measurement bias within the study population. Inter-observer variability was defined by the mean difference and standard deviations between the two observers.
Statistical Analysis
By calculating the standard deviation and mean limb ratios in the control group, Z scores were derived for limb ratios in the achondroplasia group. A paired t-test was performed to evaluate differences between right and left lower limb ratios in the control group. An unpaired t-test was performed to evaluate differences in the limb length ratios between the two populations. The null hypothesis was that no difference exists between the limb proportions in achondroplasia and the control groups.
Genetic Analysis
Where available, results from any genetic testing within the study population (i.e., achondroplasia) group were obtained. This information did not form the basis for inclusion criteria to our study, as it is not routinely tested in all cases of achondroplasia, however, was sought to help remove doubt regarding the diagnosis within outlier cases, should they be present.
RESULTS
We identified 22 infants with achondroplasia who had undergone limb radiographs with typical clinical and radiological features of the dysplasia to form our study population. One patient in the achondroplasia group did not have imaging of the whole femur on skeletal survey, and therefore, only an upper limb ratio could be measured for this single case. Hundred and fifty average statured controls were identified over a 3-year period. Full patient demographics are included in Table I.
| Achondroplasia group | Control group | |
|---|---|---|
| Total patients | 22 | 150 |
| Mean age (days) ± sd (days) | 70 ± 94 | 113 ± 88 |
| Range of ages (days) | 0–324 | 5–348 |
| Male | 12 (54.5%) | 102 (68.0%) |
Within the control group, there was no significant difference between left and right limb length ratios for upper (P = 0.1958) or lower limbs (P = 0.1651). The average difference in limb length measurements for the upper limb was −0.1 ± 0.9 mm (range: −1.4 to 2.5 mm), and for the lower limb was −0.1 ± 1.7 mm (range: −9.1 to 5.6 mm). We therefore, used the combined mean limb length for the left and right for all subsequent assessments.
Upper Limb Length Ratios
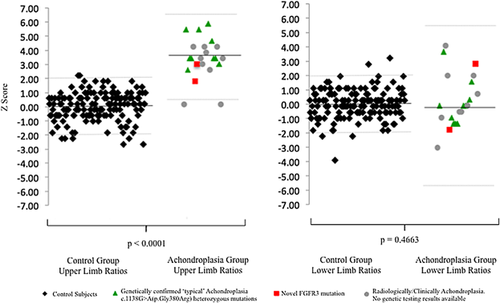
We found a statistically significant difference between the mean upper limb length ratios (radial/humeral length) in the control group versus the achondroplasia group (0.79 ± 0.02 vs. 0.87 ± 0.04, P < 0.0001).
The average Z-score for the upper limb ratios in the achondroplasia group was 3.42 ± 1.44 (range: 0.19 to 5.88, 19 (86%) patients had derived Z scores of ≥2 and ≤−2) versus those for the control group of 0 ± 1.00 (range: −2.66 to 2.22, 8(5%) patients had derived Z scores of ≥2 and ≤−2).
Lower Limb Length Ratios
No statistical significance was found in mean lower limb length ratios (tibia/femoral length) in the control group versus the achondroplasia group (0.83 ± 0.02 vs. 0.84 ± 0.04, P = 0.4663).
The average Z-score for the lower limb ratios in the achondroplasia group was 0.19 ± 1.85 (range: −3.09 to 4.06; 4 (19%) patients with Z scores ≥2 and ≤−2) versus those for the control group of 0 ± 1.00 (range: −3.93 to 3.22; 5 (3%) patients with Z scores ≥2 and ≤−2). The upper and lower limb ratios with accompanying Z scores are given in Table II.
| Group | Limb ratio, mean value ± sd (range) | Z scores, mean value ± sd (range) |
|---|---|---|
| Control group–Upper limb* | 0.79 ± 0.02 (0.72–0.84) | 0 ± 1.00 (−2.66–2.22) |
| Achondroplasia–Upper limb | 0.87 ± 0.04 (0.79–0.93) | 3.42 ± 1.44 (0.19–5.88) |
| Control group–Lower limb* | 0.83 ± 0.02 (0.74–0.91) | 0 ± 1.00 (−3.93–3.22) |
| Achondroplasia–Lower limb | 0.84 ± 0.04 (0.76–0.93) | 0.15 ± 2.81 (−3.09–4.06) |
- * In the control study group, both right and left upper and lower limb measurements were obtained for allpatients. A value for upper and lower limb ratios was then calculated from an average of the bilateral measurements.
Figure 1 demonstrates the upper and lower limb ratios for the control and achondroplasia populations with ± 2sd limits.
Inter-Observer Agreement
There was good inter-observer agreement of limb length measurements in the achondroplasia group with an average measurement difference of 0.1 mm (range: −3.3 to 6.8 mm, standard deviation = 1.4 mm).
Genetic Analysis
FGFR3 mutation sequencing was available in 12 patients, and 10 patients had the common c.1138G>A (p.Gly380Arg) heterozygous mutation. Two patients had a rare heterozygous pathogenic mutation in the extracellular domain creating cystein residues. One had the c.835A>T (p.Ser279Cys) mutation, which has been described in three other cases. Their phenotypes spanned a relatively broad range encompassing hypochondroplasia to severe achondroplasia. The other had the c.833A>G (p.Tyr278Cys) mutation, which has been reported in an antenatal presentation described as “severe hypochondroplasia.” Patients with genetic mutation information have been denoted within Figure 1. For the remaining 10 patients, mutation analysis was not available; however, in two patients the medical notes recorded a confident clinical diagnosis of achondroplasia.
DISCUSSION
Patients with achondroplasia demonstrated mesomelic shortening of their limbs with the addition of statistically significant “rhizomelic” shortening within their upper limbs, but not their lower limbs, compared with an average statured control group. The results reveal a potential inaccuracy in the medical literature regarding radiographic descriptions of limb proportions in achondroplasia.
There are two previous studies, published regarding differences in radiographic limb proportions between an achondroplastic and an average statured population [Langer et al., 1967; Nehme et al., 1976]. In one study [Langer et al., 1967], lower limb proportions in achondroplasts of various ages (34 subjects aged 0–14 years old, of which 6 were aged under 1 years of age) were compared with average statured published standards, not a locally derived control population. Although they reported rhizomelic shortening of the lower limbs, we were concerned that published standards may not be the most accurate comparison between different populations. In addition, the authors commented upon subjective marked rhizomelic shortening in the upper limbs, however, numerical data for upper limb ratios were not calculated.
The second study [Nehme et al., 1976] analyzed differences in limb length measurements rather than ratios between an achondroplasia population, and published average statured standards. The authors of this paper concluded the presence of lower limb rhizomelic shortening, due to the fact that femoral lengths were a greater number of standard deviations (average sd = 8; sd range = −10.1 to −6.4) below the published standards than tibial lengths (average sd = 7; sd range = −9.5 to −4.1) in 18 patients aged between 0–18 years old. A test of statistical significance was not performed, and upper limb ratios were not calculated as radiographs were not obtained of this region.
Our study included both upper and lower limb ratios within a locally derived control group, and an achondroplastic population. All measurements for our achondroplasia patients were calculated by two observers blinded to each other's findings, whereas, previous studies have only employed one observer. We therefore, consider our method to be a more accurate way of assessing for differences between the populations.
A further strength to our study is that it includes information regarding patient genetic analysis. Interestingly within our results, we identified two patients with rare FGFR3 mutations who were not outliers within the study population. This suggests that our findings regarding limb length proportions within our achondroplasia population need not necessarily be confined to those with “typical” FGFR3 mutations. Alternatively, within the study group, there were two patients with upper limb length ratios lying further than two standard deviations from the mean who had not undergone genetic analysis for their diagnosis. Although one may argue that the diagnosis of achondroplasia may be disputed in these cases without genetic analysis, in the presence of typical clinical features, and supportive radiographic findings, genetic testing is not always undertaken to confirm a clinical diagnosis in the United Kingdom. It is possible that in certain cases, there may not be rhizomelic shortening of the upper limbs in infants at all.
Our findings help to correct a widely published medical myth relating to the radiographic description of achondroplasia. This study may be of use to radiologists, less experienced in assessing skeletal dysplasias, who could potentially misinterpret “rhizomelic limb shortening” as a pre-requisite in diagnosing achondroplasia. Conversely, the presence of true, severe rhizomelic shortening may suggest diagnoses other than achondroplasia, which may be overlooked if there is too much emphasis placed upon rhizomelic shortening in achondroplasia. We have not demonstrated whether this information can be used in altering patient management and may not assist experienced clinicians, and radiologists in diagnosis.
There were several potential limitations to our study. The relatively small sample size of patients with achondroplasia led to wide confidence intervals within which limb length ratios may lie. Nevertheless, the significant differences between mean values of upper limb ratios do still lend validity to our conclusions. In addition, we analyzed our study group at one point in time, namely at diagnosis. Our study did not investigate whether the limb length proportions vary over time or with increasing age.
The use of post-mortem skeletal imaging as an average statured (“normal”) control population could be also argued as not reflective of “live” age-matched control subjects. We attempted to counter this by correlating all control subjects with their autopsy results to confirm none of the causes of death were associated with altered bone growth. Each post-mortem skeletal survey was also double reported by two consultant paediatric radiologists to exclude underlying radiographic bone abnormalities. Given that very few healthy live children have complete imaging of both upper and lower limbs, and the ethical implications in subjecting normal patients to increased radiation burden for research purposes, analysis of post-mortem imaging was felt to be the most realistic method by which to derive a control population. The use of published standards may have been an alternative measure (as adopted by previous studies), however, published “normal” limb ratios [Robinow and Chumlea, 1982] may not be applicable to the 21st century paediatric population.
We also confirmed cases of achondroplasia by radiographic rather than genetic testing. In practice, although genetic testing would usually be offered it is not considered essential in reaching a diagnosis owing to the constellation of multiple characteristic features of the condition, and therefore, our case inclusion reflects those of normal practice.
Furthermore, differences in skeletal survey imaging for dysplasia diagnoses nationwide [Offiah and Hall, 2003] (as oppose to those for post-mortem skeletal surveys) meant that not all imaging for our achondroplasia population was standardized (for example, the laterality of upper and lower limbs, use of radiographic equipment, and number of images differed between subjects). This factor resulted in one patient without full imaging of either femur, meaning a lower limb ratio for this case could not be derived. For this reason, two observers were employed in measuring limb lengths in the achondroplasia group to prove that despite such variation, measurement results were consistent.
Future work on the subject of achondroplasia and limb proportions may include analysis of a larger population sample, and exploration for reasons behind the differences in upper and lower limb proportions. It is already speculated that the effects of FGFR3 mutations are responsible for relative overgrowth of the fibula [Matsui et al., 2001; Stanley et al., 2002; Lee et al., 2007] and this may hold true for the differences in limb proportions also.
To conclude, patients with achondroplasia demonstrate mesomelic limb shortening, with the addition of rhizomelic shortening of the upper, but not the lower limbs. The results reveal a potential inaccuracy in the medical literature regarding radiographic descriptions of limb proportions in achondroplasia.
ACKNOWLEDGMENTS
Dr. Owen J. Arthurs is funded by a National Institute of Health Research (NIHR) Clinician Scientist Fellowship. The views expressed are those of the authors and are not necessarily those of the National Health Service, the NIHR, or the Department of Health.