Further delineation of FKBP14-related Ehlers–Danlos syndrome: A patient with early vascular complications and non-progressive kyphoscoliosis, and literature review
Abstract
FKBP14-related Ehlers–Danlos syndrome (EDS) is an extremely rare recessive connective tissue disorder described for the first time in 2012 by Baumann and coworkers. The causal gene, FKBP14, encodes a member of the F506-binding family of peptidyl-prolyl cis-trans isomerases. The paucity of patients described so far makes this disorder poorly defined at clinical level. Here, we report an additional pediatric patient, who is compound heterozygous for a recurrent and a novel FKBP14 mutation, and compare his phenotype with those available in literature. This evaluation confirms that kyphoscoliosis (either progressive or non-progressive), myopathy, joint hypermobility, and congenital hearing loss (sensorineural, conductive, or mixed) are the typical features of the syndrome. Since the patient showed a severe cardiovascular event in childhood and atlantoaxial instability, this report expands the phenotype of the disorder and the allelic repertoire of FKBP14. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Ehlers–Danlos syndromes (EDS) are clinically and genetically heterogeneous connective tissue disorders (CTDs) characterized by involvement of skin, joints, ligaments, vasculature, and internal organs [Steinmann et al., 2002]. Six EDS types are included in the Villefranche nosology [Beighton et al., 1998] and several rare variants were more recently delineated [Colombi et al., 2015]. Among these, Baumann et al. [2012] reported a rare recessive variant of EDS characterized by joint hypermobility, progressive kyphoscoliosis, myopathy, and sensorineural hearing loss due to recessive mutations in FKBP14. This gene encodes a member of the F506-binding family of peptidyl-prolyl cis-trans isomerases found in the lumen of the endoplasmic reticulum, where it is thought to catalyze cis-trans-isomerization of peptidyl-prolyl peptide bonds and to accelerate protein folding, particularly of procollagens [Galat, 2003]. To date, eight patients with FKBP14-related EDS from seven independent families have been described: five pediatric (≤12 years), one young woman (16 years), and two adults (48 and 42-year-old) [Baumann et al., 2012; Aldeeri et al., 2014; Murray et al., 2014].
Here we report an 8-year-old patient affected by FKBP14-related EDS due to compound heterozygosity for a recurrent and a novel FKBP14 mutation and compare the proband's clinical presentation with the features of the patients available in literature. Our patient presented with vascular rupture at 6 years and showed atlantoaxial instability, never described before in FKBP14-related EDS, thus expanding the clinical phenotype of this rare disorder.
CLINICAL REPORT
The patient, an 8-year-old Italian male, was born from healthy non-consanguineous parents at 36 weeks by cesarean for cephalic presentation. At birth, measurements were within normal range (weight 3rd–15th centile, length 50th centile). Perinatal presentation was abnormal, with severe muscle hypotonia and poor suck. Newborn hearing screening with Auditory Brainstem Response Audiometry suggested congenital bilateral profound hearing loss. He underwent transtympanic drains due to impaired middle ear ventilation; audiometric testing, performed subsequently, revealed a partial improvement of hearing loss. At age 8 years, he had mixed hearing loss of mild degree. Gross motor milestones were delayed; he walked without help at the age of 2.6 years. Non-progressive and non-surgical kyphoscoliosis was first observed at 6 months and successfully treated with lumbar orthosis. At the age 2 years, EDS VIA was excluded by lysyl pyridinolines to hydroxylysyl pyridinoline (LP/HP) urinary ratio analysis, which resulted within the normal range. A flexion extension radiograph of the cervical spine performed at the age 6 years revealed an asymptomatic atlantoaxial instability with enlarged atlanto-epistropheal distance in flexion (Fig. 1A, a,b).
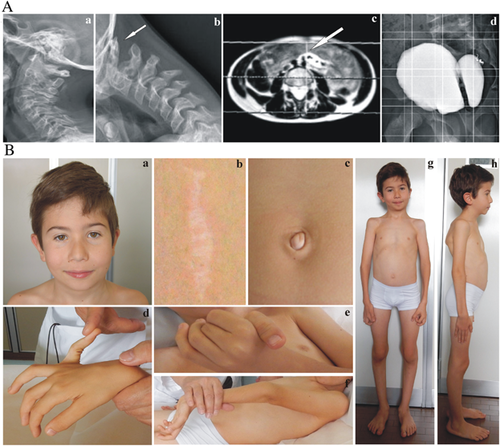
Given generalized muscle hypotonia, at age 3 years primary myopathy was suspected and histopathological examination of muscle biopsy specimens revealed changes in muscle fiber diameters, which were first linked to a nemaline myopathy. ACTA1, TPM2, and TPM3 molecular analysis involved in nemaline myopathy types 3, 4, and 1, respectively were negative. Muscle hypotonia slowly improved with psychomotor rehabilitation programs. At age 6 years, the patient was admitted to emergency unit for acute abdomen caused by left hypogastric artery pseudoaneurysm rupture, safely treated with endovascular procedure (Fig. 1A, c). Thoracic and abdominal magnetic resonance angiography (MRA) performed after the vascular event did not show other arterial malformations, such as ectasia, aneurysm, pseudoaneurysm, or tortuosity. Echocardiogram was normal. At age 8 years, an asymptomatic arachnoid cyst of the dorsal spine was successfully surgically removed. Diagnostic evaluation for voiding dysfunction, in particular urinary retention, lead to the diagnosis of a large bladder diverticulum by ultrasound; uroflussometry performed at the age of 8 revealed detrusor dyssynergia of I–II grades (Fig. 1A, d). The patient used braces for ankle instability from 3 to 5 years. At age 6, vitamin D supplementation was initiated due to radiological findings of osteopenia.
The patient came to our attention at age 8 years; clinical examination revealed normal stature (1.27 m, 25th–50th centile) and weight (22 kg, 10th–25th centile), generalized muscle hypotonia with myopathic gait, palpebral and umbilical skin redundancy, epicanthal folds, periorbital creases, light blue sclerae, long philtrum, micrognathia, bifid uvula, an atrophic scar on the knee, an enlarged scar after muscle biopsy, joint hypermobility according to Beighton score (6/9), high palate, winged scapulae, kyphoscoliosis of mild degree, mild pectus excavatum, bilateral valgus elbow, valgus knee, and pes planus (Fig. 1B).
MOLECULAR CHARACTERIZATION
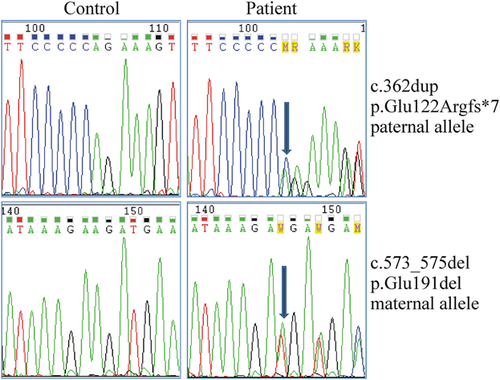
After informed consent was obtained from the parents, molecular characterization was performed on genomic DNA purified from peripheral blood leukocytes using standard procedures. All exons and intron-flanking regions of FKBP14 were PCR amplified by using optimized genomic primer sets, as previously described [Baumann et al., 2012]. PCR products were purified with ExoSAP-IT (USB Corporation, Cleveland, OH) followed by bidirectional sequencing with the BigDye Terminator v1.1 Cycle Sequencing kit on an ABI 3130XL Genetic Analyzer (Applied Biosystems, Carlsbad, CA). FKBP14 sequence analyses revealed compound heterozygosity for the paternal and recurrent c.362dup (p.Glu122Argfs*7) and the maternal novel c.573_576del (p.Glu191del) mutations (Fig. 2), thus confirming the diagnosis of FKBP14-related EDS.
DISCUSSION
The clinical presentation of the patient reported here was strongly suggestive for FKBP14-related EDS, as he showed all the typical signs of this extremely rare CTD, that is, kyphoscoliosis, muscle hypotonia, and hearing loss. In addition to these cardinal features, at age 6 years, he had a left hypogastric artery dissection, and atlantoaxial instability. Clinical features and biochemical findings of the nine patients affected by FKBP14-related EDS, proven by molecular analysis, are summarized in Tables I and II, respectively. Differential diagnosis should take into account other EDS types with early onset/congenital kyphoscoliosis and myopathic signs, that is, EDS kyphoscoliotic type, CHST14-related EDS, in addition to CTDs with vascular involvement including EDS vascular type, Marfan and Loeys–Dietz syndromes, and myopathies, particularly those with joint laxity, such as Ullrich congenital dystrophy and Bethlem myopathy. The hearing loss distinguishes FKBP14-related EDS from these disorders. Moreover, FKBP14-related EDS differs from EDS kyphoscoliotic type by the normal ratio of urinary LP/HP crosslinks [Rohrbach et al., 2011], from CHST14-related EDS by the absence of congenital contracture of thumbs and fingers, and from the above mentioned CTDs with vascular involvement by the absence of a typical facial appearance and visceral rupture, marfanoid habitus, craniosynostosis, and widespread aortic/arterial aneurysms [Colombi et al., 2015]. Absence of respiratory muscle failure, improvement of muscle hypotonia with age and presence of an unspecific myopathic pattern on electrophysiological and muscle biopsy studies are useful aids to differentiate FKBP14-related EDS form primary myopathies [Baumann et al., 2012].
All the reported FKBP14-related EDS patients showed the cardinal features of the disease, that is, hearing loss, early-onset kyphoscoliosis, and myopathic signs, though with high variability (Table I). Hearing impairment can vary from sensorineural (6/8), to conductive (1/8), or mixed (present case) (Table I). In our patient, because of the mixed origin of this sign, hearing improved after transtympanic drains. Kyphoscoliosis is noted at a mean of 12 months (range 2–18 months) and it can be non-progressive (three patients including ours) or progressive (6). Orthotic treatment seemed successful in case of non-progressive kyphoscoliosis, as in our patient; progressive kyphoscoliosis required a surgical approach. Myopathic signs include muscle hypotonia and atrophy (9/9), poor head control in infancy (7/7), and delayed motor development (9/9) (Table I). Muscular weakness seems to regress with age and all of the subjects-but one-became able to walk at the mean age of 33 months. The outcome is very variable and the final ability to walk ranges from myopathic gait, impossibility of walking without aids, to a motor self-sufficiency from 200 m to 1 km (Table I). Muscle biopsy always shows pathological results with a broad spectrum of findings; creatine kinase generally results within the normal range (5/7) and electromyographic pattern can vary with age (Table II).
| P1 Family A Present case | P2 Family B Baumann et al. [2012] | P3 Family B Baumann et al. [2012] | P4 Family C Baumann et al. [2012] | P5 Family D Baumann et al. [2012] | P6 Family D Baumann et al. [2012] | P7 Family F Baumann et al. [2012] | P8 Family G Murray et al. [2014] | P9 Family H Aldeeri et al. [2014] | |
|---|---|---|---|---|---|---|---|---|---|
| Sex/Age | M/8 | M/16 | F/48 | F/11 | F/16 | M/11 | F/3 | M/42 | M/3 |
| Family history | + | + | |||||||
| Perinatal presentation | Severe hypotonia | Severe hypotonia | Severe hypotonia | Severe hypotonia | Hypotonia | Hypotonia | Hypotonia | Hypotonia | Hypotonia |
| Poor sucking | Poor sucking | Poor sucking | Severe poor sucking | Poor sucking | Poor sucking | Poor sucking | |||
| Muscle atrophy | Low oxygen saturation | ||||||||
| Age at diagnosis | 7 | 16 | 48 | 11 | 16 | 11 | 3 | 42 | 3 |
| FACIAL DYSMORPHISMS | |||||||||
| Epicanthal folds | + | na | na | na | na | na | na | + | + |
| Hypotelorism | na | na | na | na | na | na | na | + | |
| Square nasal root | na | na | na | na | na | na | na | + | |
| Uvula abnormalities | + bifid | na | na | na | na | na | na | na | |
| Micrognathia | + | na | na | na | + | na | + | na | |
| Long-narrow face | na | na | na | na | na | na | + | na | |
| OCULAR | |||||||||
| Myopia | + | + | + | + | + | + | na | ||
| Hypermetropia | + | na | na | ||||||
| MUCOCUTANEOUS | |||||||||
| Hyperextensible skin | + | + | + | + | + | + | na | + | |
| Soft texture | + | + | + | + | + | + | + | na | |
| Umbilical skin redundancy | + | na | na | na | na | na | na | na | + |
| Follicular hyperkeratosis | + | + | + | + | na | na | |||
| Hernia | + inguinal | + umbilical | + umbilical | + umbilical | na | ||||
| Defective scars | + | + | + | na | na | ||||
| Bluish sclerae | + | + | na | na | |||||
| SKELETAL | |||||||||
| Height (centile) | Normal for age (25–50th) | Normal for age (10–25th) | Normal for age (50th) | Normal for age (10th) | Short stature (<3rd) | Normal for age (10–25th) | Normal for age (10–25th) | Short stature (<3rd) | na |
| Palate deformities | + high | + cleft | + cleft | + high | na | ||||
| Kyphoscoliosis | + | + | + | + | + | + | + | + | na |
| Age of onset | 6 mth | 2 mth | 6 mth | 4 mth | 18 mth | 12 mth | 18 mth | 12 mth | |
| Evolution | non progressive | progressive | progressive | progressive | progressive | non progressive | non progressive | progressive | |
| Treatment | lumbar orthosis | lumbar orthosis | surgery, 11 y | surgery, 4 y | lumbar orthosis and surgery,12 y | surgery, 3 and 10 y | |||
| Pectus deformity | + mild excavatum | + secondary to scoliosis | + carenatum | ||||||
| Flat feet | + | + | + | + | + | + | + | na | na |
| Club foot | + left | + left | + bilateral | na | |||||
| Joint hypermobility (Beighton score) | + | + | + | + | + | + | + | + | + |
| 6/9 | 6/9 | 6/9 | 8/9 | 6/9 | 9/9 | 9/9 | na | 8/9 | |
| Osteopenia | Na | + | + | + | + | + | + | + | + |
| Fractures | + | ||||||||
| NEUROMUSCULAR | |||||||||
| Muscle hypotonia | + | + | + | + | + | + | + | + | + |
| Muscle atrophy | + | + | + | + | + | + | + | + | + |
| Poor head control in infancy | + | + | + | + | + | + | + | na | na |
| Walking independently/Actual walking | + 30 mth | + 30 mth | + 30 mth | + 48 mth | + 24 mth | + 48 mth | + na | + 48 mth | |
| myopathic gait, low autonomy | independently walking up to 1 km | independently low autonomy | myopathic gait wheelchair after walking 200 m | na | myopathic gait | wheelchair | myopathic gait | myopathic gait | |
| Weakness improvement | + | + | + | + | + | + | + | + | na |
| NEUROLOGICAL | |||||||||
| Arachnoid cyst | + | na | na | ||||||
| Subdural hygroma | + | na | na | ||||||
| CARDIOVASCULAR | |||||||||
| Valvular abnormalities | na | + | + | ||||||
| Patent ductus arteriosus | na | + | |||||||
| Aortic aneurysm/Pseudoaneurysm (age, site) | + | na | + | ||||||
| 6 y, left hypogastric artery pseudoaneurysm | 41 y, left hypogastric artery | ||||||||
| Vascular dissection (age, site) | + | + | |||||||
| 6 y, left hypogastric artery | celiac artery | ||||||||
| VISCERAL | |||||||||
| Bladder diverticulum | + | + | na | na | na | + | na | na | na |
| Neurogenic bladder | + | na | na | na | na | na | na | na | |
| Rectal prolapse | + | na | na | na | na | na | na | na | na |
| OTOLOGIC | |||||||||
| Congenital, non-progressive hearing loss | + mixed | + sensorineural | + sensorineural | + sensorineural | + conductive | + sensorineural | + sensorineural | + sensorineural | na |
| Recurrent otitis | + | na | na | na | na | na | na | + | na |
- na, not available.
| P1 Family A Present case | P2 Family B Baumann et al. [2012] | P3 Family B Baumann et al. [2012] | P4 Family C Baumann et al. [2012] | P5 Family D Baumann et al. [2012] | P6 Family E Baumann et al. [2012] | P7 Family F Baumann et al. [2012] | P8 Family G Murray et al. [2014] | P9 Family H Aldeeri et al. [2014] | |
|---|---|---|---|---|---|---|---|---|---|
| Sex/Age | M/8 | M/16 | F/48 | F/11 | F/16 | M/11 | F/3 | M/42 | M/3 |
| LABORATORY | |||||||||
| Creatin kinase | Normal | Normal | Slighty elevated | Normal | Normal | Slightly elevated | Normal | na | na |
| Urinary crosslinks | Normal | Normal | Normal | Normal | Normal | Normal | Normal | na | na |
| NEUROMUSCOLAR EVALUATION | |||||||||
| Nerve conduction | Normal | Normal | Normal | Normal | Normal | Normal | Normal | na | na |
| Electromyography age and results | 1 mth, Normal | 3 mth, Normal | 6 y, Normal | 4 mth, Normal | 1 y, Myopathic | na | na | na | 3 y, Myopathic |
| 15 y, Myopathic | 30 y, Myopathic | 2 y, Normal | |||||||
| Muscle CT/MRI age and results | 7 mth | 15 y | na | na | 12 y | 12 y | na | na | na |
| Normal | Normal | signal abnormalities in rectus femoris, vastus lateralis, and soleus | signal abnormalities in rectus femoris, vastus lateralis, medial head of gastrocnemius, soleus, paraspinal | ||||||
| Muscle biopsy age, site and results | 2 y, quadriceps, chronic muscle suffering compatible with congenital myopathy; EM: variability of myofibrillar diameter, fiber hypotrophy, roundish fiber microgranulation with nemaline rods, increased endomysial connective tissue intermyofibrillar alteration | 2 y, quadriceps, Irregular oxidative enzymes; EM: focal myofibrillar rearrangement | 2 y, quadriceps, Marked fiber atrophy; 4 y, na, marked atrophy, fatty tissue proliferation; 7 y, anterior tibial, myopathic fatty tissue proliferation; 30 y paraspinal, mildly myopathic atrophic fibers | 4 mth, quadriceps, mildly myopathic; 4 y paraspinal, areas with fiber atrohy, slightly increased intrafusal fat; 6 y paraspinal, areas with central activity defects of oxidative enzymes; EM: focal myofibrillar rearrangement | 1 y, quadriceps, mildly myopathic; 6 y, na, myopathic; 12 y dorsal myopathic with increased variation of fiber diameter; EM: sarcomeres bifurcation, small zone of Z-band streaming and disorganized myofibrils | 1 y, quadriceps, myopathic | 1 y, quadriceps, mildly myopathic | na | na |
| Brain MRI age and results | 1 y | na | na | na | na | 9 y | na | 36y , Normal | na |
| Normal | Mild white matter atrophy | 41 y, mild/moderate volume loss minimal periventricular and subcortical white matter hypersynthesis | |||||||
| CARDIOVASCULAR EVALUATION | |||||||||
| Echocardiogram | 7 y | 13 y | na | na | 13 y | na | na | 41 y | na |
| Normal | Normal | Normal | Tricuspid valve insufficiency | Normal | |||||
| Carotid US doppler | na | na | na | na | na | na | na | 36 y | 3 y |
| Normal | Patent ductus arteriosus | ||||||||
| Thoraco-abdominal MRA age and results | 7 y | na | na | na | na | na | na | na | na |
| Normal, regular embolization of left hypogastric artery | |||||||||
| OTHERS | |||||||||
| Spirometry | na | na | na | na | na | na | na | 41 y | na |
| Obstructive/restrictive lung disease | |||||||||
| Flexion extension X-rays of cervical spine | 6 y | na | na | na | na | na | na | na | na |
| Atlantoaxial instability with enlarged atlanto-epistropheal distance in flexion | |||||||||
- na, not available.
In addition to these hallmarks, FKBP-14-related EDS patients show a variable constellation of signs due to connective tissue impairment affecting ocular, mucocutaneous, skeletal, nervous central, and cardiovascular systems (Table I). The cardiovascular risk in FKBP14-related EDS is not well defined (Table I). Vascular complications are described in P8 (Table I), who presented a celiac artery pseudoaneurysm rupture at the age of 41, and in the older sister of P3 (Table I), likely affected but without molecular confirmation, who died due to unspecified aortic rupture at age 12 years. Celiac artery pseudoaneurysm rupture was observed in our patient at age 6 (Fig. 1). Atlantoaxial instability is reported here for the first time, the other patients presented uncomplicated joint hypermobility without recurrent dislocations/sprains or chronic pain (mean value of Beighton score 7/9) (Table I). Facial dysmorphisms are not always described and a facial “gestalt” is not recognizable, some patients had epicanthal folds (3/3), micrognathia (3/4), hypotelorism (1), square nasal root (1), or long-narrow face (1) (Table I). Height is generally within the normal range, but at lower level (10th–25th centile in 4 of 8), two patients had short stature (lower than third centile) (Table I). Tissue fragility in FKBP14-related EDS may lead to large bladder diverticulum (Fig. 1) or rectal prolapse (present case).
In conclusion, our report supports many of the clinical data available in literature for FKBP14-related EDS, confirming the variability observed for the cardinal features. In addition, this report describes a potentially life-threatening vascular complication in early pediatric age and atlantoaxial instability, suggesting the need for FKBP14-related EDS patients of tailored follow-up that includes cardiovascular monitoring, that is, cerebral, thoracic and abdominal MRA, and cervical dynamic radiograph. Further studies are needed to better delineate the phenotype of FKBP14-related EDS.
ACKNOWLEDGMENTS
The authors thank the patient's parents for their cooperation during the diagnostic process of their son.