Homozygous HOXB1 loss-of-function mutation in a large family with hereditary congenital facial paresis
Abstract
Hereditary congenital facial paresis (HCFP) belongs to the congenital cranial dysinnervation disorders. HCFP is characterized by the isolated dysfunction of the seventh cranial nerve and can be associated with hearing loss, strabismus, and orofacial anomalies. Möbius syndrome shares facial palsy with HCFP, but is additionally characterized by limited abduction of the eye(s). Genetic heterogeneity has been documented for HCFP as one locus mapped to chromosome 3q21-q22 (HCFP1) and a second to 10q21.3-q22.1 (HCFP2). The only known causative gene for HCFP is HOXB1 (17q21; HCFP3), encoding a homeodomain-containing transcription factor of the HOX gene family, which are master regulators of early developmental processes. The previously reported HOXB1 mutations change arginine 207 to another residue in the homeodomain and alter binding capacity of HOXB1 for transcriptional co-regulators and DNA. We performed whole exome sequencing in HCFP-affected individuals of a large consanguineous Moroccan family. The homozygous nonsense variant c.66C>G/p.(Tyr22*) in HOXB1 was identified in the four patients with HCFP and ear malformations, while healthy family members carried the mutation in the heterozygous state. This is the first disease-associated HOXB1 mutation with a likely loss-of-function effect suggesting that all HOXB1 variants reported so far also have severe impact on activity of this transcriptional regulator. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Facial palsy at birth is usually a unilateral compression neuropathy from forceps application during delivery that recovers spontaneously [Eng, 1971]. However, hereditary congenital facial paresis (HCFP) is a developmental anomaly characterized by isolated dysfunction of the facial nerve (CN VII) [Verzijl et al., 2003]. The presence of isolated bilateral facial weakness is often but incorrectly referred to as Möbius syndrome (MBS) (MIM 157900) [Towfighi et al., 1979]. Refinement of the minimal diagnostic criteria for MBS led to the definition of “congenital, uni- or bilateral, nonprogressive facial weakness and limited abduction of the eye(s)” [Verzijl et al., 2003; Carta et al., 2011; Mackinnon et al., 2014]. Recently, heterozygous de novo mutations in PLXND1 or REV3L have been identified in individuals with classical MBS [Tomas-Roca et al., 2015].
MBS and HCFP can be accompanied by additional clinical manifestations, such as hearing loss, orofacial anomalies, limb malformations, and musculoskeletal system defects [Verzijl et al., 2003; Terzis and Anesti, 2011]. Linkage analysis in large families with autosomal dominant HCFP revealed one locus at chromosome 3q21-q22 (HCFP1; MIM 601471) [Van Der Zwaag et al., 2005; Verzijl et al., 2005] and a second at 10q21.3-q22.1 (HCFP2; MIM 604185) [Verzijl et al., 1999]. Sequencing of candidate genes in the delineated intervals did not reveal any pathogenic mutation in individuals with HCFP or MBS [Van Der Zwaag et al., 2002, 2004].
In 2012, Webb and co-workers reported the first gene, HOXB1, to be associated with congenital facial palsy and additional features, such as sensorineural hearing loss, strabismus, esotropia, feeding difficulties, and midface retrusion (HCFP3; MIM 614744) [Webb et al., 2012]. Brain imaging revealed facial nerve agenesis and bilateral abnormal tapering of the basal turn of the cochlea in one patient. In two sibling pairs, both of German American descent, the homozygous HOXB1 mutation c.619C>T predicting the amino acid substitution p.(Arg207Cys) was detected [Webb et al., 2012]. HOXB1 encodes one of 39 homeodomain-containing transcription factors of the highly conserved homeobox gene family. This class of transcriptional regulators is of major importance for early developmental morphogenetic processes, especially the anterior–posterior patterning of the developing embryo [Mallo and Alonso, 2013]. To date, mutations in ten HOX genes have been found to cause different human disorders [Quinonez and Innis, 2014]. Molecular modeling and in vitro functional analysis predicted the arginine-to-cysteine change at position 207 in the homeodomain to diminish binding of HOXB1 to transcriptional co-regulators and DNA, thereby altering transcriptional activity of HOXB1 [Webb et al., 2012]. Another homozygous missense mutation in HOXB1, also affecting arginine 207 [c.620G>A/p.(Arg207His)] was found in a Turkish girl with bilateral facial weakness, left esotropia, left ptosis, and midface retrusion. Auditory brainstem response test revealed normal results and MRI scans showed no structural anomalies. Similar to the other four reported individuals with HOXB1 mutation, the HCFP-affected girl showed full oculomotor motility. The p.(Arg207His) substitution has been suggested to have a less severe impact on HOXB1 transcriptional activity than p.(Arg207Cys), correlating with a milder phenotype [Uyguner et al., 2015]. Identification of point mutations in HOXB1 predicting substitution of the same amino acid residue leaves open the question whether these alterations are loss-of-function alleles. As the phenotype in the five reported HOXB1-mutation positive individuals resembles that of Hoxb1 knockout mice, which display absence or reduction in size of facial motor nerve and abnormal contralateral vestibuloacoustic neuron migration [Goddard et al., 1996; Studer et al., 1996], a loss-of-function mechanism mediated by the HOXB1 germline missense mutations has been proposed [Quinonez and Innis, 2014].
In a family with two consanguineous marriages and four individuals affected by HCFP over two generations, we identified a novel homozygous one-base-pair substitution in the HOXB1 gene leading to introduction of a premature stop codon. To the best of our knowledge, this germline mutation most probably represents the first loss-of-function HOXB1 allele associated with HCFP to date.
SUBJECTS AND METHODS
Human Subjects
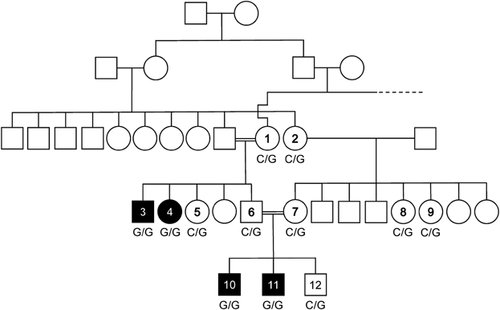
Clinical data, blood, and/or cheek swab samples were obtained from 12 members of the family with informed consent, including consent to use photographs. Consent procedures were approved by the Ethics Committees of the Medical Chamber of Hamburg (reference number PV3802). The consanguineous family originated from Morocco. Affected individuals were 7 (subject 10), 11 (subject 11), 26 (subject 3), and 34 (subject 4) years old at last follow-up (Fig. 1). The two affected brothers were clinically assessed by experienced pediatricians.
Whole Exome Sequencing (WES) and Data Analysis
DNA was isolated from leukocytes or buccal cells by standard procedures. We pooled equal amounts of DNA from the four affected individuals 3, 4, 10, and 11 (Fig. 1) and used this single genomic DNA probe to perform targeted enrichment and massively parallel sequencing as described previously [Kortüm et al., 2015; Van Rahden et al., 2015]. Whole exome enrichment was performed using Nextera® Exome Enrichment Kit (62 Mb) (Illumina, San Diego, CA) according to the manufacturer's protocols. Captured libraries were loaded onto the HiSeq 2500 platform (Illumina). For variant analysis, the workflow recommended by the Genome Analysis Toolkit (GATK) was applied. By using the Burrows–Wheeler Aligner (BWA v0.7.12) reads were aligned to the human reference genome (UCSC GRCh37/hg19). Presumed PCR duplicates were removed using Picard's MarkDuplicates. GATK (v3.3) was used for realignment of sequences encompassing small insertions and deletions (indels) and base quality recalibration. Indels and single nucleotide variants were identified by means of the GATK Haplotype Caller and Unified Genotyper algorithms. Variants were functionally annotated and filtered against public databases (dbSNP138, 1,000 Genomes, and ExAC) and in-house databases using AnnoVar (v2015-03-22). Exonic sequence alterations and intronic variants at exon–intron boundaries ranging from −5 to +5, which were clinically associated, with unknown frequency or minor allele frequency (MAF) <0.5% and not present in the homozygous state in public databases were retained. Exome read-depth metrics and variant filter strategy are summarized in Table SI.
Variant Validation and Segregation Analysis
Sanger sequencing was performed to confirm the HOXB1 variant identified by WES in the four affected individuals and to test segregation of this variant in the eight healthy family members. Primer pair and PCR condition are available on request. The amplicon was directly sequenced using the ABI BigDye Terminator Sequencing Kit (Applied Biosystems, Darmstadt, Germany) and an automated capillary sequencer (ABI 3500; Applied Biosystems). Sequence electropherograms were analyzed using the Sequence Pilot software (JSI Medical Systems, Kippenheim, Germany). Mutation nomenclature refers to GenBank mRNA reference sequence NM_002144.3.
RESULTS
Clinical Data
Patient 11 (Figs. 1 and 2) presented to us at age 6 years because of diabetes mellitus type 1. We observed facial paresis in this boy. He and his brother (patient 10 in Figs. 1 and 2) had been clinically diagnosed with MBS at birth. Except of strabismus in patient 11, eye movements were unaffected in both brothers. Patients 10 and 11 had a history of feeding difficulties and speech delay requiring oro-motor and speech therapy. In both individuals, taste discrimination, salivation and lacrimation were intact, as was general sensation over the concha of the ear and skin behind the auricle. Stapedius reflex was normal bilaterally.
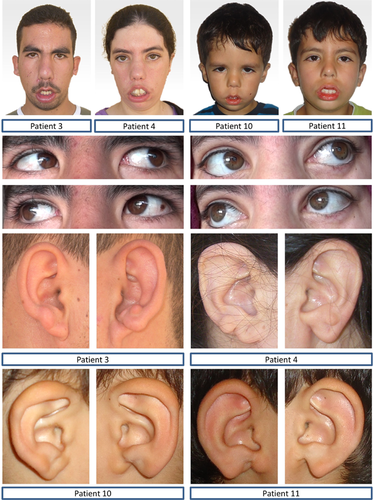
Clinical work-up revealed that the paternal uncle (patient 3 in Figs. 1 and 2) and the paternal aunt (patient 4 in Figs. 1 and 2) of patients 10 and 11 who both lived in Morocco also had seventh nerve palsy but remained undiagnosed. The four affected individuals of the family were born to healthy consanguineous parents, exhibited typical signs of HCFP and presented with auricular malformations at birth (Fig. 2), which were absent in HCFP-unaffected family members known to M.V. in person (data not shown). As individuals 3, 4, 10, and 11 had full eye motility (Fig. 2), they did not meet the clinical criteria for MBS.
Additional clinical characteristics of all affected individuals were midface retrusion, an upturned nose, smooth philtrum, lagophthalmos, mild oral dysfunction secondary to facial paresis, feeding problems, swallowing difficulties, palatal weakness, dysarthria, and speech delay. In all affected individuals, craniofacial dysmorphism included epicanthic folds, flat nasal bridge, external ear defects (Fig. 2), and variable cone-shaped lateral incisors. External auditory canal and skeletal abnormalities, delay of motor development, attention disorder, and vascular defects were absent in the four affected individuals (data not shown).
Brain MRI in patients 10 and 11 identified bilateral facial nerve hypoplasia but no cochlea abnormality. Auditory brainstem response testing of patient 11 revealed bilateral moderate high frequency hearing loss with normal absolute latencies of waveforms. Distortion product otoacoustic emissions (DPOAE) were absent in patient 11, supporting abnormal cochlear function that resulted in moderate speech delay and prescription of hearing aids. Similarly, patient 10 showed absent DPOAE and mild hearing loss. Individuals 3 and 4 could not be tested.
Clinical features of the affected family members are summarized in Table I and compared to previously reported patients with HOXB1 mutation.
| Present family | Webb et al. [2012] | Uyguner et al. [2015] | |
|---|---|---|---|
| Molecular findings | |||
| NM_002144.3 | c.66C>G | c.619C>T | c.620G>A |
| NP_002135.2 | p.(Tyr22*) | p.(Arg207Cys) | p.(Arg207His) |
| Craniofacial findings | |||
| Masked facies | 4/4 | 4/4 | 1/1 |
| Bilateral facial weakness | 4/4 | 4/4 | 1/1 |
| Full oculomotor motility | 4/4 | 4/4 | 1/1 |
| Esotropia/esophoria | 1/4 | 4/4 | 1/1 (left) |
| Ptosis | 0/4 | 0/4 | 1/1 (left) |
| Smooth philtrum | 3/4 | 2/4 | 0/1 |
| Midface retrusion | 4/4 | 4/4 | 1/1 |
| Upturned nasal tip | 4/4 | 4/4 | 1/1 |
| Low-set ears | 1/4 | 3/4 | 1/1 |
| Micrognathia | 0/4 | 2/4 | 0/1 |
| Ear malformations | 4/4 | nd | nd |
| Other findings | |||
| Feeding difficulties | 4/4 | 2/4 | 0/1 |
| Sensorineural hearing loss | 2/2 | 4/4 | 0/1 |
| Abnormal cochlear function | 2/2 | 2/2 | 0/1 |
| Cochlear structural defect | 0/2 | 1/1 | nd |
- nd, no data.
Molecular Data
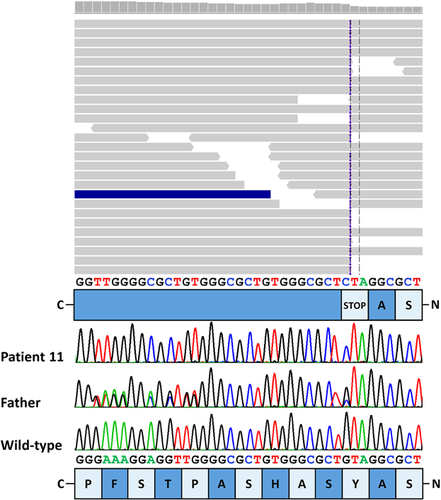
Consanguinity of the parents of patients 3 and 4 as well as 10 and 11 suggested autosomal recessive inheritance and a shared homozygous variant in the four HCFP-affected individuals over two generations (Fig. 1). We therefore pooled DNA of the four patients and performed WES on one DNA sample. After filtering of WES data, we obtained a single private homozygous 9-bp insertion in exon one of the HOXB1 gene, originally annotated as c.65_66insGAGCGCCCA (Fig. 3). We Sanger-sequenced part of HOXB1 exon one in the two affected sib pairs and corrected the identified indel as follows: at codon 22, a C-to-G change was found to lead to introduction of a premature stop codon [c.66C>G/p.(Tyr22*)] which was followed by a 9-bp duplication, c.74_82dupACAGCGCCC [p.(His25_Ala27dup)], a known polymorphism (ss102025026) (Fig. 3). The nonsense mutation was absent from public databases (dbSNP, the Exome Variant Server, the 1000 Genomes Project, and the ExAC Browser). Segregation analysis revealed the HOXB1 variant c.66C>G in the heterozygous state in eight healthy family members available for this study (Fig. 1). Together, homozygosity of the truncating HOXB1 mutation p.(Tyr22*) in all HCFP-affected individuals and heterozygosity in healthy family members strongly indicated that this loss-of-function HOXB1 allele underlies HCFP in the Moroccan family.
DISCUSSION
We report here the first nonsense mutation in the HOXB1 gene to cause HCFP type three in a large family of Moroccan descent. The c.66C>G/p.(Tyr22*) mutation in HOXB1 is predicted to encode a C-terminal truncated protein without the DNA-binding homeodomain (amino acid residues 207–262). However, as the C-to-G change is located 521 nucleotides upstream of the exon 1-exon 2 junction in the HOXB1 transcript, we propose nonsense-mediated mRNA decay to occur in HOXB1-expressing cells of the four Moroccan individuals with HCFP [Fatscher et al., 2015], likely leading to a null allele. The functional consequences of the two known missense variants p.(Arg207Cys) and p.(Arg207His) have been reported to affect binding of HOXB1 to transcriptional co-regulators and DNA, however, a severe impact on transcriptional activity of HOXB1 could not be demonstrated in vitro [Webb et al., 2012; Uyguner et al., 2015]. Nonetheless, it has been suggested that these mutations cause loss of function due to phenotypic similarities with Hoxb1-deficient mice [Webb et al., 2012; Uyguner et al., 2015]. Interestingly, patients reported here exhibited additional clinical features, such as malformations of the external ear, which have not been observed in mice with disrupted hoxb1 gene but in hoxa1/hoxb1 double knockout mice [Goddard et al., 1996; Studer et al., 1996; Gavalas et al., 1998]. Thus, it is possible that another disorder segregates with HCFP3 in the Moroccan family. Therefore, we visually examined the WES reads for variants in HOXA1, HOXA2, HOXB2, and HOXB3 but did not identify any rare or private variant in these genes. Although we cannot exclude a variant segregating with the auricular malformation in the family, a combined phenotype of HCFP and external ear anomalies associated with the HOXB1 p.(Tyr22*) variant appears more likely. Moreover, co-occurrence of HCFP and external ear anomalies seems probable as the second pharyngeal arch contributes to both the development of the facial nerve and the external ear [Wright, 1997; Frisdal and Trainor, 2014].
Taken together, by discovering the nonsense mutation p.(Tyr22*) in family members with HCFP and auricular malformations which have not been reported in the five previously described individuals with HCFP3 [Webb et al., 2012; Uyguner et al., 2015], we assume incomplete loss-of-function for previously described biallelic HOXB1 variants, whereas the nonsense mutation most likely represents a null allele.
Facial palsy has been suggested to have an intrauterine etiology or a genetic cause [Laing et al., 1996; Oystreck et al., 2011]. In particular, lack of a positive family history may lead to a misdiagnosis of facial palsy. In patients with persistent facial nerve palsy and additional clinical abnormalities, the suspicion of a genetic disorder should be raised. The recent definition of minimum diagnostic criteria of MBS should facilitate accuracy in clinical evaluation and properly subdivide patients in groups diagnosed for example with MBS or HCFP [Mackinnon et al., 2014]. According to the diagnostic criteria for classic MBS, all affected individuals of the Moroccan family reported here were initially misdiagnosed as having MBS [Mackinnon et al., 2014]. Although one causative gene has yet been identified for HCFP [Webb et al., 2012] and two for MBS [Tomas-Roca et al., 2015], the majority of patients with MBS or HCFP failed to get a molecular genetic diagnosis to date [Mackinnon et al., 2014; Tomas-Roca et al., 2015]. Indeed, only 1% of individuals with HCFP with or without additional clinical features were found to carry a HOXB1 mutation [Uyguner et al., 2015], implying genetic heterogeneity.
The recent identification of de novo mutations in the genes PLXND1 and REV3L in patients with MBS shed first light into the pathomechanism of this cranial dysinnervation disorder [Tomas-Roca et al., 2015]. PLXND1 is a member of the plexin protein family which bind to semaphorins and regulate neuronal migration [Oh and Gu, 2013]. REV3L is the catalytic subunit of DNA polymerase zeta required for mutagenic replication of damaged DNA [Makarova and Burgers, 2015]. Although the two proteins are implicated in completely different pathways, deficiency of each of the two genes in mice causes hypoplasia of the facial motor nucleus [Tomas-Roca et al., 2015]. Similarly, in Hoxb1 knockout mice the facial branchiomotor (FBM) nucleus is reduced in size or completely absent and there is reduction in the motor component of the facial nerve [Goddard et al., 1996; Studer et al., 1996; Arenkiel et al., 2004]. While Hoxb1 functions in the formation and maintenance of FBM neuron circuitry [Arenkiel et al., 2004], PLXND1 and REV3L regulate motor neuron migration and proliferation at the FBM nucleus, respectively [Tomas-Roca et al., 2015]. These data show that mutations in the three disease genes similarly disrupt motor neuron migration to the FBM nucleus. In line with this, bilateral facial paresis and no weakness of the abducens nerves in a patient with REV3L mutation indicate that HCFP and MBS are allelic conditions [Tomas-Roca et al., 2015], and mutations in REV3L and PLXND1 may also cause HCFP.
In individuals affected by HCFP careful clinical examination is mandatory. Genetic testing in early infancy can guide diagnostic interventions and obviate unnecessary examinations. The availability of effective tools for genetic testing and knowledge of genes causing HCFP should encourage pediatricians to give genetic testing high priority, especially in newborns and infants with suspected HCFP.
ACKNOWLEDGMENTS
We are grateful to all family members who participated in this study. We thank Inka Jantke and Dennis Zorndt for skillful technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (KO 4576/1-1 to F.K. and KU 1240/5-1 to K.K.).