An emerging, recognizable facial phenotype in association with mutations in GLI-similar 3 (GLIS3)
Abstract
Neonatal diabetes and hypothyroidism (NDH) syndrome was first described in 2003 in a consanguineous Saudi Arabian family where two out of four siblings were reported to have presented with proportionate IUGR, neonatal non-autoimmune diabetes mellitus, severe congenital hypothyroidism, cholestasis, congenital glaucoma, and polycystic kidneys. Liver disease progressed to hepatic fibrosis. The renal disease was characterized by enlarged kidneys and multiple small cysts with deficient cortico-medullary junction differentiation and normal kidney function. There was minor facial dysmorphism (depressed nasal bridge, large anterior fontanelle, long philtrum) reported but no facial photographs were published. Mutations in the transcription factor GLI-similar 3 (GLIS3) gene in the original family and two other families were subsequently reported in 2006. All affected individuals had neonatal diabetes, congenital hypothyroidism but glaucoma and liver and kidney involvement were less consistent features. Detailed descriptions of the facial dysmorphism have not been reported previously. In this report, we describe the common facial dysmorphism consisting of bilateral low-set ears, depressed nasal bridge with overhanging columella, elongated, upslanted palpebral fissures, persistent long philtrum with a thin vermilion border of the upper lip in a cohort of seven patients with GLIS3 mutations and report the emergence of a distinct, probably recognizable facial gestalt in this group which evolves with age. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Taha et al. [2003] described the first consanguineous Saudi Arabian family in which two of four siblings had permanent neonatal diabetes associated with intrauterine growth retardation (IUGR), congenital hypothyroidism, facial anomalies, congenital glaucoma, hepatic fibrosis, and polycystic kidneys, described as NDH (Neonatal Diabetes and Hypothyroidism) syndrome. Genome wide linkage analysis and sequencing of candidate genes performed on this family by Senée et al. [2006] identified a homozygous frameshift mutation (c.1873dupC, previously reported as 2067insC) in GLIS3 which is likely to result in transcript degradation by nonsense mediated decay (NMD). Both children with this mutation died in infancy. A child born subsequently in this family died of the same condition prior to confirmatory genetic testing [Habeb et al., 2012]. Senée et al. [2006] described two further families with mutations in GLIS3. The first harboured a homozygous 426-kb deletion, which encompassed the SLC1A1 gene and part of GLIS3. The affected offspring in the second family carried a homozygous 149-kb deletion that included a portion of GLIS3 as well; the region common to both deletions mapped to the known start codon of GLIS3. Patients in both these families presented with a milder phenotype with the absence of renal or liver disease. Additional features in these and subsequent patients described include intrauterine growth retardation (IUGR), developmental delay, and congenital glaucoma [Senée et al., 2006]. We recently published a large series of 12 patients worldwide with mutations in GLIS3 thus expanding the clinical spectrum of abnormalities resulting from disruption of Glis3 function. These include exocrine pancreatic insufficiency, osteopenia, fractures with delayed fracture healing, craniosynostosis, hiatus hernia, congenital cardiac disease, splenic and pancreatic cysts, and choanal atresia [Dimitri et al., 2015].
A facial phenotype has been cited in patients with mutations in GLIS3 [Taha et al., 2003; Senée et al., 2006]. Facial dysmorphology previously described includes a large and squared shape of the face, with a thin curved nose and previous reports suggest that the facial features attenuate with growth. To date, the facial phenotype in patients with GLIS3 mutations has not been described in detail and the degree of consistency in these features between patients has not been reported. We thus reviewed the features in seven patients with GLIS3 mutations in whom written consent was obtained for publication of images to determine whether a consistent facial phenotype was present in patients with mutations in GLIS3 and whether there was a recognizable facial gestalt.
MATERIALS AND METHODS
The study was conducted in accordance with the Declaration of Helsinki principles with informed parental consent given on behalf of children. Clinical information was provided by the referring clinicians, from clinical notes and subsequently using a questionnaire circulated to referring clinicians to gain further information. Consent was received from parents to publish photographs of facial features resulting from mutations in GLIS3.
Genetic Analysis
GLIS3 gene mutations were identified by PCR amplification (primer sequences available on request) and sequence analysis of exons 1–11 by comparison with the reference sequence NM_001042413. Exon 1 is non-coding (the 5′ UTR) and the start codon is located within exon 2. The effect of coding variants on the protein was investigated in silico using the bioinformatic tool ALAMUT (Interactive Biosoftware, Rouen, France). When failure of PCR amplification occurred, suggesting a homozygous deletion, parental samples were investigated by real-time quantitative PCR on an ABI 7900 (TaqMan assay with SYBR Green detection) and the copy number of exons 1–11 was determined by the 2−ΔΔCt method.
Patient 4 was analyzed for all the known neonatal diabetes genes using a targeted next generation assay [Ellard et al., 2013]. Mutations identified by this assay were confirmed by Sanger sequencing.
RESULTS
Table I describes the nucleotide and predicted protein changes of the GLIS3 mutations identified in the patients studied. Table II describes the clinical features in the cohort reported here and the homozygous GLIS3 mutation results (six with deletions and one with a homozygous missense mutation). Patients 2 and 3 are siblings aged 7 and 2.4 years, respectively. A consistent facial phenotype is seen at a younger age in patients, that is, bilateral low-set ears, depressed nasal bridge with upturned nose, prominent eyes, long philtrum with a thin vermilion border of the upper lip (Fig. 1). With age, rather than being attenuated as described in previously published literature [Senée et al., 2006], it appears to evolve into a more recognizable facial gestalt consisting of bilateral low-set ears, depressed nasal bridge with overhanging columella, elongated, upslanted palpebral fissures, persistent long philtrum with a thin vermilion border of the upper lip. This demonstrates the consistent facial phenotype in patients with GLIS3 mutations. The oldest patient in this series is 7 years of age and appears to have a distinct facial gestalt (Patient 1).
| Patient number | Exon | Mutation | Nucleotide change | In silico prediction | Current age (years) |
|---|---|---|---|---|---|
| 1 | 1–2 | Exons 1–2 del/exons 1–2 del | c.-?_388+?del/c.-?_388 +?del | Pathogenic | 7.1 |
| 2 | 1–4 | Exons 1–4 del/exons 1–4 del | c.-?_1710+?del/c.-?_1710 +?del | Pathogenic | 7.0 |
| 3 | 1–4 | Exons 1–4 del/exons 1–4 del | c.-?_1710+?del/c.-?_1710+?del | Pathogenic | 2.4 |
| 4 | 5–9 | Exons 5–9 del/exons 5–9 del | c.1711-?_2473+?del/c.1711-?_2473+?del | Pathogenic | 4 |
| 5 | 9–11 | Exons 9–11 del/exons 9–11 del | c.2298-?_2657+?del/c.2298-?_2657+?del | Pathogenic | Died at 6 years |
| 6 | 4 | p.His561Tyr/p.His561Tyr | c.1681C>T/c.1681C>T | Pathogenic | 5.3 |
| 7 | 1–2 | Exons 1–2 del/exons 1–2 del | c.-?_388+?del/c.-?_388+?del | Pathogenic | 5 |
| Patient number | Current age (years) | Birth weight (g) | IUGRa | Gestation (weeks) | Ethnicity | Gender | Consanguineous | PND Onset∼ (days) | Congenital Hypothyroidism | Liver disease | Kidney disease | Exocrine pancreatic disease | Congenital Glaucoma | Skeletal Disease | Developmental delay | Facial dysmorphism | Other features |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (#2) | 7.1 | 1,170 | Yes | 35 | Bangladeshi | Female | Yes | 3 | Yes | Yes | Yes | Yes | No | Osteopenia, fractured ribs, scoliosis | Yes, mild | Yes | No |
| 2 (#3a) | 7.0 | 1,430 | Yes | 35 | Caucasian | Male | No | 4 | Yes | Yes | Yes | Yes | No | No | Yes, mild | Yes | BSN PDA pancreatic cyst |
| 3 (#3b) | 2.4 | 2,020 | Yes | 38 | Caucasian | Male | No | 2 | Yes | Yes | Yes | Yes | No | No | Yes, mild | Yes | Pancreatic and splenic cyst BSNHL |
| 4 (#4) | 4 | 1,750 | Yes | 34 | Arab | Female | Yes | 2 | Yes | Yes | Yes | No | No | No | Yes, mild | Yes | No |
| 5 (#6) | Died at 6 years | 1,530 | Yes | 37 | African-American | Female | Unknown | 7 | Yes | Yes | Yes | No | Yes | Sagittal craniosynostosis | Yes, mild | Yes | No |
| 6 (#10) | 5.3 | 973 | Yes | 31 | Kurdish | Male | Yes | 31 | Yes | Yes | Yes | No | Yes | No | No | Yes | PDA |
| 7 (#11) | 5 | 1,730 | Yes | 39 | Arab | Male | Yes | 19 | Yes | No | Yes | No | Yes | No | No | Yes | Ostium secundum ASD |
- #In brackets are Patient numbers as detailed in Dimitri et al. [2015].
- a Birth weight <10th centile for gestational age; PND, Permanent neonatal diabetes; BSNHL, Bilateral sensori-neural hearing loss; PDA, Patent ductus arteriosus; ASD, Atrial Septal Defect.
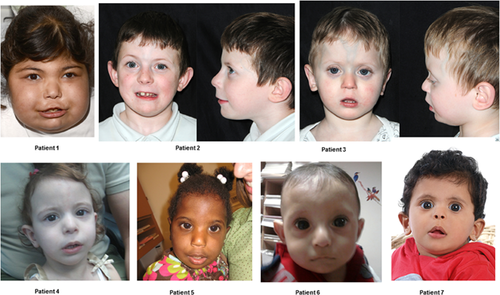
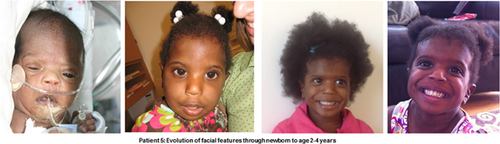
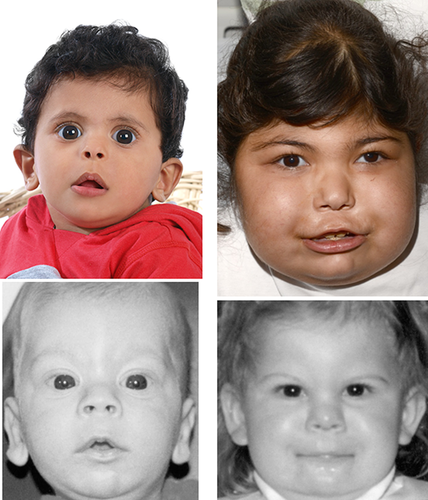
Figure 2 demonstrates the progression of the facial phenotype with age in the same patient (Patient 5) from newborn, aged 2 up to 4.3 years while Figure 3 demonstrates the striking resemblance of patients in this series to the images previously published by Senée et al. [2006] (reproduced with relevant permission from Nature Publishing Group).
DISCUSSION
GLIS3 is a member of the GLI-similar zinc finger protein family encoding for a nuclear protein that maps to chromosome 9p24.3-p23 (OMIM *610192) [Kim et al., 2003]. Mutations in GLIS3 have been reported in association with Neonatal diabetes mellitus and hypothyroidism syndrome (NDH syndrome—OMIM #610199). GLIS3 is expressed in early embryogenesis and plays a critical role as both a repressor and activator of transcription by interacting with a specific nucleotide sequence, known as the Gli response element (GLI-RE) in the promoter region of target genes [Kim et al., 2003; Beak et al., 2008]. Glis proteins contain a DNA binding domain consisting of five C2H2-type zinc finger motifs that are critical for nuclear localization. Two major GLIS3 transcripts from the 11 exon gene have previously been described—7.5 kb and smaller (0.8–2.0 kb); the 7.5-kb transcript is strongly expressed in pancreas, thyroid, and kidney with smaller transcripts predominantly expressed in liver, kidney, eye, heart, and skeletal muscle [Senée et al., 2006]. The cardinal feature of mutations in GLIS3 is the concomitant presentation of neonatal diabetes and congenital hypothyroidism although recently a patient with a compound heterozygous mutation in GLIS3 who did not develop hypothyroidism was reported [Dimitri et al., 2015].
Neonatal diabetes is likely to result from the disrupted interaction of GLIS3 with key regulatory genes in pancreatic embryogenesis including ONECUT1 and NEUROGENIN3 (NEUROG3) [Poll et al., 2006; Kang et al., 2009a,b; Kim et al., 2012]. GLIS3 expression persists beyond the embryonic period promoting beta cell proliferation and regulating insulin gene expression through binding to GLI-RE on the INS gene [Yang et al., 2013]. The proposed mechanism by which biallelic pathogenic variants in GLIS3 causes a multi-system phenotype has been reported elsewhere [Senée et al., 2006; Dimitri et al., 2015].
GLIS3 is expressed during embryonic face development [Kim et al., 2003], which may help in part to explain the dysmorphic facial features observed in these patients. In studies of mouse embryos, whole mount in situ hybridization from stage e6.5 to e14.5 was used to determine the temporal and spatial patterns of GLIS3 expression during development. Facial expression of GLIS3 was greatly increased from e11.5 to e12.5, and expression was mesenchymal in origin. GLIS3 is also expressed in a dynamic pattern during eye development and at e8.75, GLIS3 transcripts were evident in the region of the otic vesicles. Given the commonality of GLIS3 expression with bone morphogenic proteins (BMPs) and other members of the TGFβ superfamily, interactions between BMPs and GLIS3 have been speculatively proposed as a possible underlying mechanism that is important for facial development. However, beyond this, there is no clear genetic interaction to explain the common facial dysmorphology observed in patients with mutations in GLIS3.
Table II provides clinical features in patients reported in this series demonstrating the multi-system phenotype associated with mutations in GLIS3. Patients 1–5 presented with mild developmental delay with age at walking ranging between 13 and 21 months of age and first word between 15 and 18 months of age. Patients 6–7 were not reported to have developmental delay. As evident from the patient images, all the patients in this series appear to have a similar facial gestalt with bilateral low-set ears, prominent eyes with upslanted palpebral fissures, depressed nasal bridge, long philtrum, and thin vermilion border of the upper lip (Fig. 1). The facial features become more evident and established with advancing age (Fig. 2). The patients reported in this series appear to have similar facial features as the patients reported by Senée et al. [2006]. The two siblings from the third family in the reported series—NDH3-3 and NDH3-4, at age 6 months and 2 years, respectively were said to have characteristic facial features (Fig. 3). In our cohort, Patient 7 in particular appears to have a very similar facial dysmorphology to the patient at 6 months of age (NDH3-3—Senée et al., 2006), while Patient 1 in our series appears to share the same facial gestalt as the patient at 2 years of age (NDH3-4—Senée et al., 2006). Two of the patients in this series, Patients 4 and 6, have mutations which affect only the GLIS3 gene (an interstitial deletion and a missense mutation), supporting the hypothesis that the facial features observed result from the GLIS3 defect alone and not from the deletion of contiguous genes. The patients not included in this series (including a 36-year-old adult and 7-month-old child) that were previously reported have the same facial dysmorphism as described above with prominent eyes, upslanted palpebral fissures, long philtrum, and thin vermilion border of the upper lip but as we have been unable to obtain written consent from patient and their families, we have been unable to include them in this report [Dimitri et al., 2015—Patients 1 and 9].
In combination with the other features in NDH, it is important to recognize the facial gestalt in this group of patients in order to direct targeted genetic testing and provide genotype–phenotype correlation. This is particularly important as making an early diagnosis and instituting therapeutic intervention is crucial given the course of disease pathology in NDH syndrome. In conclusion, we report a common and emerging recognizable facial gestalt in patients with GLIS3 mutations that are not attenuated with age. Further case reports of this nature are crucial in elaborating the common facial phenotype in NDH and attributing a consistent facial phenotype to this genetic condition.
ACKNOWLEDGMENTS
We thank the families for their participation in this report.