Fetal alcohol spectrum disorders and assessment of maxillary and mandibular arc measurements
Abstract
Fetal alcohol spectrum disorders (FASD) comprise a range of physical differences and neurologic deficits from prenatal alcohol exposure. Previous studies suggest that relative maxillary growth deficiency can accompany FASD. Using the Fetal Alcohol Syndrome Epidemiologic Research (FASER) database, we investigated how maxillary and mandibular arcs and the ratio between them differ between FASD and non-FASD individuals. First, we established normative values for maxillary and mandibular arcs and maxillary-to-mandibular arc ratio. In our control group (545 males, 436 females), mean maxillary and mandibular arcs for males/females were 24.98/24.52 cm and 25.91/25.35 cm, respectively. The ratio was 0.9643 and 0.9676 for males and females, respectively. We then evaluated the effect of microcephaly, short stature, and low weight (<10th centile), individually on arcs in controls. Generally, arcs were reduced significantly but the ratio did not differ. We compared our controls to 138 male and 135 female FASD cases. We noted a significant difference in arcs in male and female groups, but not the ratio. We compared non-FAS controls with reduced growth parameters to similar cases with FASD. We did not find a significant difference in arc or ratio measurements. Therefore, we conclude the effect of prenatal alcohol exposure on maxillary and mandibular arc measurements is primarily on overall facial growth and less on asymmetric growth of the maxilla relative to the mandible, at least using this technique. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
The term fetal alcohol syndrome (FAS) describes a recognizable pattern of abnormal physical and intellectual development due to prenatal alcohol exposure. The manifestations of physical and neurological symptoms and signs accompanying prenatal alcohol exposure display a continuum, and the collective defects have been termed Fetal Alcohol Spectrum Disorders (FASD).
FASD are considered the leading cause of non-hereditary mental retardation and a major cause of intellectual disability worldwide [May and Gossage, 2001]. In the United States, FAS is estimated to occur at two to seven per 1,000, and FASD at 2–5% [May et al., 2009, 2014], and international investigations have demonstrated a prevalence as high as 68.0–89.2 per 1,000 in South Africa [May et al., 2007]. The annual cost of treatment for FASD was estimated to be $4 billion in 1998, including the costs of raising an affected child, as well as other indirect effects on families and society [Senturias, 2014]. Therefore, tools for accurate diagnosis of FASD are critical in order to identify and begin prompt treatment of affected individuals, as well as to eliminate consequences of misdiagnosis.
The diagnosis of FASD is based on specific physical and neurodevelopmental characteristics. Physical characteristics include cardinal facial features (a smooth philtrum, thin vermillion border of the upper lip, and short palpebral fissures) and a reduction in key growth parameters (reduced height, weight, or head circumference) [Abel, 1998]. Other observed features include midface hypoplasia, micrognathia, epicanthal folds, ear anomalies, or a low nasal bridge [Sampson et al., 1997]. Neurodevelopmental characteristics include a range of intellectual disability, such as problems with learning, attention, memory, social interactions [May and Gossage, 2001], school performance, or higher level receptive and expressive language function [Stratton et al., 1996]. Also, central nervous system defects, including microcephaly, partial or complete agenesis of the corpus callosum, or cerebellar hypoplasia can be seen [Stratton et al., 1996].
FASD is split into several categories, including full FAS, partial FAS, alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD), based on the physical and neurological features as described by [Hoyme et al., 2005]. Confirmed maternal alcohol consumption is not required for a diagnosis in those diagnostic categories which require specific dysmorphology to be present (FAS and PFAS); whereas, in the other two categories (ARND and ARBD), confirmation of maternal alcohol use in pregnancy is required. No dose–effect relationship has been established for the amount of alcohol consumed and the presence of fetal defects [Landgraf et al., 2013].
The mechanism by which alcohol affects fetal development is complex. One way alcohol is thought to alter facial development is through deleterious effects on neural crest cell migration [Goodlett and Horn, 2001]. These cells help form the brachial arches, which are involved in the development of facial cartilage and bone, and peripheral nerves of the head and face [Chai et al., 2000; Goodlett and Horn, 2001]. Animal models have shown neural crest cells to be sensitive to environmental changes, and the disruption of these cells may produce the typical facies seen in FASD [Chen and Sulik, 1996; Goodlett and Horn, 2001; Sulik, 2005; Shen et al., 2013]. Altered neural crest cell migration is suggested to contribute to microcephaly and subsequent decreased brain growth [Naidoo et al., 2006], as well as to alterations in development of the maxilla and mandible [Chai et al., 2000; Sulik, 2005]. Other mechanisms proposed for alcohol-induced changes to the developing fetus include altered growth factor and gene regulation, changes in intercellular calcium, and signaling cascades, decreased placental support to the fetus, or impaired retinoic acid production [Goodlett and Horn, 2001; Sulik, 2005]. These teratogenic mechanisms may act independently or simultaneously to create the spectrum of features seen in FASD.
Midface hypoplasia is a known feature of FASD [Naidoo et al., 2006], shown to be present in animal models [Lipinski et al., 2012] as well as 3D modeling of FASD individuals [Suttie et al., 2013]. However, the etiology of midface hypoplasia has been debated. Proposed non-skeletal causes suggest the perception of midface hypoplasia is based on the relationship of other facial features to one another [Gir et al., 1989; Riekman, 1984]. Proposed skeletal causes include restriction of facial growth and subsequent retrusion of the maxilla [Frias et al., 1982], or underdevelopment of maxilla with a concurrent longer face secondary to mandible gonial angle, and ramus length [Naidoo et al., 2006]. For both non-skeletal and skeletal causes, the relationship between the maxilla and mandible is important in determining midface hypoplasia. To help assess this variable objectively, the maxillary and mandibular arcs can be measured on physical exam. Direct anthropometric evaluations, as studied by Moore et al. [2002], have been used to consistently and quickly identify features of FASD to assist in diagnosis. Direct anthropometric measurements, when used in conjunction with complete FASD evaluations, can help connect observed clinical traits and their anatomical basis [Moore et al., 2002]. For example, Moore et al. [2002] found there to be a measured decrease in all facial depths in FASD individuals, but that the midface depth was decreased further than the upper or lower face, thus, supporting the appearance of midface hypoplasia.
The Fetal Alcohol Syndrome Epidemiologic Research (FASER) database contains information from extensive screenings of school-age children for FASD. For this report, we analyzed variables reported in the FASER database to investigate the effects of prenatal alcohol exposure on facial growth. Specifically, we looked at the maxillary and mandibular arcs, and the ratio between them, to compare how arc growth differs between FASD and non-FASD individuals. Further, we questioned if alcohol exposure primarily affects growth of the maxillary and mandibular arcs, or the overall growth of the face in affected individuals.
MATERIALS AND METHODS
The FASER studies [Hoyme et al., 2005] evaluated first grade students (5–9 years old) for FASD from locations in South Africa, Italy, and the United States by first screening for reduced growth parameters. Those individuals with height, weight, or head circumference <10th centile were further evaluated with a dysmorphology assessment. Growth parameters were established by local growth charts when available, and by CDC growth charts within the United States, and WHO growth charts outside of the United States. Evaluation assessed cardinal facial features (via the Clarren–Astley lip-philtrum guide) [Astley and Clarren, 1996] and palpebral fissure length, as well as noting midface hypoplasia, flat nasal bridge, anteverted nose, prognathism, and maxillary and mandibular arc lengths among other traits [Hoyme et al., 2005]. Individuals were then classified as non-FAS, or further evaluated for FASD by neuropsychological evaluations and maternal interviews.
A diagnosis of full FAS was based on (i) two of three cardinal facial features; (ii) growth restriction of height or weight <10th centile; and (iii) evidence of central nervous system abnormalities via occiptofrontal head circumference (OFC) <10th centile or a structural brain anomaly. A diagnosis of partial FAS required (i) two of three cardinal facial features; (ii) growth restriction of height or weight <10th centile; and (iii) OFC <10th centile and/or behavioral, or cognitive abnormalities [May et al., 2007]. A diagnosis of ARND was made in those children with OFC <10th centile, or via specific behavioral or cognitive abnormalities. ARBD individuals were not studied in our analysis due to insufficient data, and a lack of clarity as to how ARBD fits within the spectrum. Confirmed maternal alcohol use during pregnancy was not required from diagnoses of FAS and PFAS, but was required for a diagnosis of ARND.
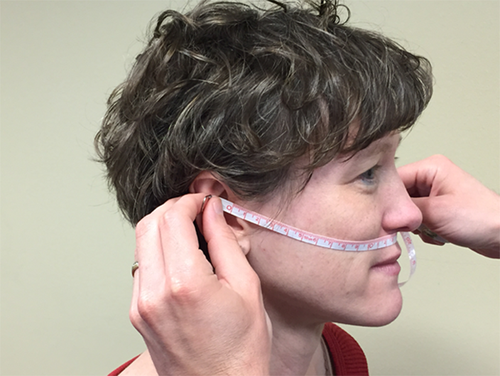
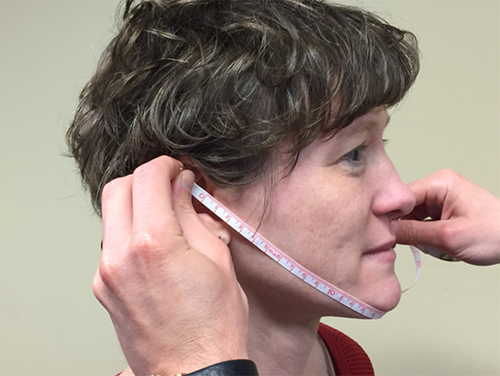
Arc measurements were made with a flexible measuring tape, ruled in millimeters. The maxillary arc was measured from the anterior notch of one ear (the superior point where the tragus meets the helix) across the upper lip (passing just under the point where the nasal septum meets the lip) to the anterior notch on the contralateral ear (Fig. 1). The mandibular arc was measured from the anterior notch on one ear across the mental protuberance (the “tip” of the chin) to the anterior notch on the contralateral ear (Fig. 2).
To evaluate the effect of alcohol on the maxillary and mandibular arc lengths and the ratio between them, we first established normative arc measurements. These were taken from individuals with a diagnosis of non-FASD, and height, weight, and OFC >10th centile (n = 544 male, 436 female). We then established our control population, comprising non-FASD individuals whose height, weight, and OFC were not necessarily >10th centile (n = 741 male, 647 female). Our case population consisted of individuals diagnosed with full FAS, partial FAS, or ARND (n = 138 male, 135 female).
We used Stata statistical software for data analysis. For the normative values, we reported the mean, standard deviation, and 95% tolerance intervals for male and female maxillary arc, mandibular arc, and the ratio between them. Then, using a paired-T test, we compared the means for arc lengths and the arc ratio for the non-FASD control population and the normative values, using a significant P-value of <0.05. We divided the control population by reduced growth characteristics (microcephalic with OFC <10th centile, short stature with height <10th centile, and low weight with weight <10th centile), and compared them to normative values.
We then evaluated the mean arc measurements and ratio in FASD cases against non-FASD controls, using separate comparisons for full FAS, partial FAS, ARND, and all FASD cases combined. Finally, we analyzed the effects of reduced growth parameters by comparing microcephalic FASD cases (OCF <10th centile) against microcephalic non-FASD controls (OFC <10th centile), as well as normocephalic FASD cases (OFC >10th centile) against normocephalic non-FASD controls (OFC >10th centile).
RESULTS
In the normative population (n = 545 males, 436 females), the mean maxillary and mandibular arcs for males/females were 24.98/24.52 cm and 25.91/25.35 cm, respectively. The maxillary-to-mandibular arc ratio was 0.9643 and 0.9676 for males and females, respectively (Table I).
| Data source | N | Mean | Std. dev. | 95% Tolerance Interval for 95% of values |
|---|---|---|---|---|
| Males | ||||
| Maxillary | 544 | 24.98 | 1.18 | 22.54–27.42 |
| Mandibular | 545 | 25.91 | 1.22 | 23.39–28.43 |
| Ratio | 544 | 0.9643 | 0.0237 | 0.9153–1.013 |
| Females | ||||
| Maxillary | 436 | 24.52 | 0.94 | 22.57–26.47 |
| Mandibular | 436 | 25.35 | 1.08 | 23.11–27.59 |
| Ratio | 436 | 0.9676 | 0.0210 | 0.9239–1.0113 |
We compared the effect of reduced growth parameters from our non-FASD controls (n = 741 male, 647 female) to the normative values. Our reduced growth non-FASD control groups consisted of microcephalic children (n = 50 males, 61 females), where OFC <10th centile and height, and weight >10th centile (Table II), short statured children (n = 42 male, 52 female), where height <10th centile and weight, and OFC >10th centile (Table III), and low weight children (n = 19 male, 11 female), where weight <10th centile, and height and OFC >10th centile (Table IV). We found that for all reduced growth control groups, the maxillary and mandibular arcs decreased significantly when compared to normative values. The arc ratios showed no significant changes, except for low weight females, which significantly increased, and microcephalic females, which decreased but did not reach significance.
| Controls | Microcephalic | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 544 | 24.98 | 1.18 | 50 | 23.90 | 0.87 | <0.0001 |
| Mandibular | 544 | 25.91 | 1.22 | 50 | 24.77 | 0.95 | <0.0001 |
| Ratio | 544 | 0.9643 | 0.0237 | 50 | 0.9650 | 0.0179 | 0.8472 |
| Females | |||||||
| Maxillary | 436 | 24.52 | 0.94 | 61 | 23.63 | 0.66 | <0.0001 |
| Mandibular | 436 | 25.35 | 1.08 | 60 | 24.57 | 0.76 | <0.0001 |
| Ratio | 436 | 0.9676 | 0.0210 | 60 | 0.9621 | 0.0189 | 0.0530 |
| Controls | FAS cases (h.c. < 10th percentile) | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 544 | 24.98 | 1.18 | 42 | 24.48 | 0.77 | 0.0074 |
| Mandibular | 544 | 25.91 | 1.22 | 42 | 25.30 | 0.80 | 0.0015 |
| Ratio | 544 | 0.9643 | 0.0237 | 42 | 0.9677 | 0.0197 | 0.3594 |
| Females | |||||||
| Maxillary | 436 | 24.52 | 0.94 | 52 | 24.26 | 0.85 | 0.0589 |
| Mandibular | 436 | 25.35 | 1.08 | 52 | 25.00 | 0.99 | 0.0254 |
| Ratio | 436 | 0.9676 | 0.0210 | 52 | 0.9708 | 0.0168 | 0.3005 |
| Controls | FAS cases (h.c. < 10th percentile) | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 544 | 24.98 | 1.18 | 19 | 23.96 | 0.82 | 0.0002 |
| Mandibular | 544 | 25.91 | 1.22 | 19 | 24.65 | 1.11 | <0.0001 |
| Ratio | 544 | 0.9643 | 0.0237 | 19 | 0.9725 | 0.0259 | 0.1375 |
| Females | |||||||
| Maxillary | 436 | 24.52 | 0.94 | 11 | 23.47 | 1.05 | 0.0003 |
| Mandibular | 436 | 25.35 | 1.08 | 11 | 23.91 | 1.30 | <0.0001 |
| Ratio | 436 | 0.9676 | 0.0210 | 11 | 0.9826 | 0.0305 | 0.0216 |
Our next analysis compared FASD cases (n = 138 males, 135 females) to non-FASD controls (Table V). Divided by diagnosis, our FASD groups were composed of full FAS (n = 45 males, 49 females) (Table VI), partial FAS (n = 67 males, 62 females) (Table VII), and ARND (n = 26 males, 24 females) (Table VIII). When compared to non-FASD controls, we found that for all FASD diagnosis groups, both males and females showed significantly decreased maxillary and mandibular arcs. The arc ratio was not significantly altered in all comparisons. The arc ratio significantly increased in full FAS males.
| Controls | All FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 741 | 24.70 | 1.08 | 138 | 23.94 | 1.08 | <0.0001 |
| Mandibular | 741 | 25.61 | 1.26 | 138 | 24.74 | 1.18 | <0.0001 |
| Ratio | 741 | 0.9650 | 0.0231 | 138 | 0.9680 | 0.0234 | 0.1640 |
| Females | |||||||
| Maxillary | 647 | 24.24 | 0.99 | 135 | 23.48 | 0.99 | <0.0001 |
| Mandibular | 647 | 25.09 | 1.38 | 135 | 24.27 | 1.13 | <0.0001 |
| Ratio | 647 | 0.9669 | 0.0272 | 135 | 0.9678 | 0.0191 | 0.7051 |
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 741 | 24.70 | 1.08 | 45 | 23.49 | 0.91 | <0.0001 |
| Mandibular | 741 | 25.61 | 1.26 | 45 | 24.16 | 0.94 | <0.0001 |
| Ratio | 741 | 0.9650 | 0.0231 | 45 | 0.9729 | 0.0280 | 0.0292 |
| Females | |||||||
| Maxillary | 647 | 24.24 | 0.99 | 49 | 22.98 | 0.73 | <0.0001 |
| Mandibular | 647 | 25.09 | 1.38 | 49 | 23.77 | 0.97 | <0.0001 |
| Ratio | 647 | 0.9669 | 0.0272 | 49 | 0.9672 | 0.0175 | 0.9362 |
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 741 | 24.70 | 1.08 | 67 | 24.31 | 1.03 | 0.0107 |
| Mandibular | 741 | 25.61 | 1.26 | 67 | 25.14 | 1.14 | 0.0033 |
| Ratio | 741 | 0.9650 | 0.0231 | 67 | 0.9677 | 0.0218 | 0.3634 |
| Females | |||||||
| Maxillary | 647 | 24.24 | 0.99 | 62 | 23.81 | 1.01 | 0.0011 |
| Mandibular | 647 | 25.09 | 1.38 | 62 | 24.66 | 1.16 | 0.0164 |
| Ratio | 647 | 0.9669 | 0.0272 | 62 | 0.96 | 0.0171 | 0.7549 |
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 741 | 24.70 | 1.08 | 26 | 23.76 | 1.16 | <0.0001 |
| Mandibular | 741 | 25.61 | 1.26 | 26 | 24.75 | 1.27 | 0.0007 |
| Ratio | 741 | 0.9650 | 0.0231 | 26 | 0.9605 | 0.0161 | 0.3150 |
| Females | |||||||
| Maxillary | 647 | 24.24 | 0.99 | 24 | 23.65 | 1.00 | 0.0041 |
| Mandibular | 647 | 25.09 | 1.38 | 24 | 24.28 | 0.99 | 0.0041 |
| Ratio | 647 | 0.9669 | 0.0272 | 24 | 0.9743 | 0.0253 | 0.1882 |
Our next comparisons looked at FASD cases with reduced growth, specifically (n = 86 male, 97 female), to non-FASD controls with microcephaly (n = 101 male, 123 female) (Table IX). Separated by diagnosis, the microcephalic FASD groups contained full FAS (n = 45 male, 47 female) (Table X), partial FAS (n = 21 male, 30 female) (Table XI), and ARND FAS (n = 20 male, 20 female) (Table XII). For these case-control comparisons, we found that most had decreased maxillary and mandibular arcs that did not reach statistical significance, except for full FAS females, which had significantly decreased maxillary arcs. The arc ratio was non-significantly altered for most comparisons, except for full FAS males, which showed a significant increase.
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 101 | 23.74 | 0.84 | 86 | 23.59 | 0.93 | 0.2511 |
| Mandibular | 101 | 24.63 | 0.91 | 86 | 24.40 | 1.05 | 0.1023 |
| Ratio | 101 | 0.9641 | 0.0188 | 86 | 0.9675 | 0.0247 | 0.2926 |
| Females | |||||||
| Maxillary | 123 | 23.46 | 0.74 | 97 | 23.26 | 0.88 | 0.0699 |
| Mandibular | 123 | 24.51 | 2.11 | 97 | 24.05 | 1.00 | 0.0518 |
| Ratio | 123 | 0.9606 | 0.0450 | 97 | 0.9673 | 0.0197 | 0.1720 |
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 101 | 23.74 | 0.84 | 45 | 23.49 | 0.91 | 0.1096 |
| Mandibular | 101 | 24.63 | 0.91 | 45 | 24.16 | 0.94 | 0.0046 |
| Ratio | 101 | 0.9641 | 0.0188 | 45 | 0.9729 | 0.0280 | 0.0274 |
| Females | |||||||
| Maxillary | 123 | 23.46 | 0.74 | 47 | 22.94 | 0.73 | <0.0001 |
| Mandibular | 123 | 24.51 | 2.11 | 47 | 23.73 | 0.97 | 0.0166 |
| Ratio | 123 | 0.9606 | 0.0450 | 47 | 0.9672 | 0.0175 | 0.3298 |
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 101 | 23.74 | 0.84 | 21 | 23.91 | 0.79 | 0.3978 |
| Mandibular | 101 | 24.63 | 0.91 | 21 | 24.83 | 1.03 | 0.3747 |
| Ratio | 101 | 0.9641 | 0.0188 | 21 | 0.9635 | 0.0218 | 0.9004 |
| Females | |||||||
| Maxillary | 123 | 23.46 | 0.74 | 30 | 23.61 | 0.89 | 0.3267 |
| Mandibular | 123 | 24.51 | 2.11 | 30 | 24.54 | 1.00 | 0.9428 |
| Ratio | 123 | 0.9606 | 0.0450 | 30 | 0.9625 | 0.0167 | 0.8207 |
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 101 | 23.74 | 0.84 | 20 | 23.49 | 1.07 | 0.2316 |
| Mandibular | 101 | 24.63 | 0.91 | 20 | 24.49 | 1.17 | 0.5237 |
| Ratio | 101 | 0.9641 | 0.0188 | 20 | 0.9594 | 0.0159 | 0.2949 |
| Females | |||||||
| Maxillary | 123 | 23.46 | 0.74 | 20 | 23.47 | 0.96 | 0.9404 |
| Mandibular | 123 | 24.51 | 2.11 | 20 | 24.08 | 0.83 | 0.3734 |
| Ratio | 123 | 0.9606 | 0.0450 | 20 | 0.9748 | 0.0270 | 0.1721 |
Lastly, to compare with our microcephalic group analyses, we looked at normocephalic non-FASD controls (n = 640 males, 523 females) with normocephalic FASD cases (n = 52 males, 38 females) (Table XIII). When dividing normocephalic FASD controls by diagnosis, no full FAS cases were present as all are microcephalic, and too few ARND cases were available to make a comparison. However, there were normocephalic partial FAS cases (n = 46 males, 32 females) to compare to normocephalic non-FASD controls (Table XIV). The maxillary and mandibular arc measurements in these comparisons showed significantly decreased arcs, while the arc ratio was not significantly altered.
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 640 | 24.85 | 1.18 | 52 | 24.52 | 1.07 | 0.0479 |
| Mandibular | 640 | 25.76 | 1.24 | 52 | 25.31 | 1.18 | 0.0127 |
| Ratio | 640 | 0.9652 | 0.0237 | 52 | 0.9690 | 0.0212 | 0.2647 |
| Females | |||||||
| Maxillary | 523 | 24.43 | 0.95 | 38 | 24.05 | 1.04 | 0.0178 |
| Mandibular | 523 | 25.24 | 1.10 | 38 | 24.82 | 1.25 | 0.0271 |
| Ratio | 523 | 0.9684 | 0.0207 | 38 | 0.9692 | 0.0174 | 0.8187 |
| Controls | FAS cases | ||||||
|---|---|---|---|---|---|---|---|
| Data source | N | Mean | Std. dev. | N | Mean | Std. dev. | P-value |
| Males | |||||||
| Maxillary | 640 | 24.85 | 1.08 | 46 | 24.50 | 1.08 | 0.0471 |
| Mandibular | 640 | 25.76 | 1.24 | 46 | 25.27 | 1.17 | 0.0104 |
| Ratio | 640 | 0.9652 | 0.0237 | 46 | 0.9696 | 0.0217 | 0.2187 |
| Females | |||||||
| Maxillary | 523 | 24.43 | 0.95 | 32 | 24.00 | 1.10 | 0.0143 |
| Mandibular | 523 | 25.24 | 1.10 | 32 | 24.78 | 1.29 | 0.0248 |
| Ratio | 523 | 0.9684 | 0.0207 | 32 | 0.9689 | 0.0172 | 0.8892 |
DISCUSSION
When analyzing a non-FASD control group with reduced growth parameters, we found that although there were a few gender-based differences, generally the maxillary and mandibular arc measurements were significantly reduced, but the ratio did not differ from the normative values (Tables II–IV). From this, we infer that reduced growth causes the arcs to become uniformly smaller, but maintains the ratio between them.
When comparing non-FASD controls to FASD cases, we generally observed the same phenomenon, with significantly decreased arc measurements and an unchanged arc ratio. (Tables V–VIII). This implies that either prenatal alcohol exposure directly impacts maxillary and mandibular growth equally, or it impacts overall growth, which then leads to decreased maxillary and mandibular growth.
To distinguish between the two options, we matched non-FASD controls and FASD cases by reduced growth parameters, specifically microcephaly, in order to control for the variable of reduced growth. We found that the arc measurements and arc ratio did not differ significantly between these cases and controls (Tables IX–XII). From this, we conclude that the reduction of maxillary and mandibular arc lengths seen in individuals with prenatal alcohol exposure is primarily due to alcohol's effect of the growth parameters of the individual in general and less on the growth of the maxilla and mandible themselves. We have already shown that reduced growth causes a decrease in the arc measurements (Tables II–IV). If alcohol primarily affected the arc growth, we would have expected to see a further decrease in the arc measurements in the microcephalic FASD cases when compared to the microcephalic controls. These data imply that the effects of alcohol on maxillary and mandibular growth in children with FASD appear to be more generalized (at least as assessed by this tool) rather than more localized to the maxillary region as observed in laboratory animals.
The study may be limited by the small sample size available for our FASD case groups. It is our hope that the research population will continue to grow as the FASER study database is continually updated with new information, and our comparisons made here can be repeated on a larger sample size. Also, the FASER database contains information from several populations around the world, all of which were combined for the data used here. It might prove helpful to further divide the database by population and to compare their FASD facial anthropometric characteristics to controls from within their native populations. Further, the technique through which the examiners evaluate measurements of the maxillary and mandibular arcs has the potential for differences between examiners. To try and limit these differences, each examiner is trained to measure in the same way, to avoid placing tension on the measuring tape during measurements, and each patient is examined by two different examiners. However, before using these measurements of mandibular and maxillary arcs, it would be important in future studies to determine if these measurements correlates with the specific FASD diagnoses. We hope to continue to use the FASER database the investigate alcohol's effects on other characteristics seen in FASD.