A distinct X-linked syndrome involving joint contractures, keloids, large optic cup-to-disc ratio, and renal stones results from a filamin A (FLNA) mutation
Abstract
We further evaluated a previously reported family with an apparently undescribed X-linked syndrome involving joint contractures, keloids, an increased optic cup-to-disc ratio, and renal stones to elucidate the genetic cause. To do this, we obtained medical histories and performed physical examination on 14 individuals in the family, five of whom are affected males and three are obligate carrier females. Linkage analysis was performed on all but one individual and chromosome X-exome sequencing was done on two affected males. The analysis localized the putative gene to Xq27-qter and chromosome X-exome sequencing revealed a mutation in exon 28 (c.4726G>A) of the filamin A (FLNA) gene, predicting that a conserved glycine had been replaced by arginine at amino acid 1576 (p.G1576R). Segregation analysis demonstrated that all known carrier females tested were heterozygous (G/A), all affected males were hemizygous for the mutation (A allele) and all normal males were hemizygous for the normal G allele. The data and the bioinformatic analysis indicate that the G1576R mutation in the FLNA gene is very likely pathogenic in this family. The syndrome affecting the family shares phenotypic overlap with other syndromes caused by FLNA mutations, but appears to be a distinct phenotype, likely representing a unique genetic syndrome. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Heyen et al. [2008] previously described a family with an apparently new genetic syndrome. The features of the affected males included congenital contractures of the distal interphalangeal (DIP) joints, progressive stiffness of the shoulders and neck, an increased optic cup-to-disc ratio, keloids, and uric acid renal stones. Obligate female carriers also had an increased optic cup-to-disc ratio; some of these females had keloids, osteoporosis, arthritis, and degenerative disc disease. The pattern of inheritance appeared to be X-linked. The current study reevaluated the family to determine the genetic cause of their condition.
Filamin A, encoded by the 48 exon FLNA X-linked gene, is a 280 kDa cytoskeletal protein that promotes actin cross-linking and links actin filaments to membrane glycoproteins [Gorlin et al., 1990]. Filamin A is composed of 24 repeated rod domains that bind to multiple proteins and an N-terminal actin-binding domain that has two calponin homology domains. The rod domains are interrupted by two hinge regions, one between repeats 15 and 16, and another between repeats 23 and 24 [Gorlin et al., 1990; Stossel et al., 2001; van der Flier and Sonnenberg, 2001]. Filamin A is involved in mechanoprotection by providing reinforcement to the membrane cortex and preventing membrane depolarization by desensitizing stretch-activated, calcium permeable channels [Glogauer et al., 1998; Kainulainen et al., 1998]. The protein is also involved in signal transduction [Stossel et al., 2001].
Mutations in FLNA have been described in several conditions. Mutations that result in partial or complete loss of filamin A protein function result in X-linked periventricular heterotopia (OMIM 30049) characterized by well-controlled seizures, normal intelligence, periventricular nodules along the margins of the lateral ventricles, and variable extra cerebral features [Fox et al., 1998; Glogauer et al., 1998; Kainulainen et al., 1998; Sheen et al., 2001; Parrini et al., 2006; Jefferies et al., 2010]. The condition usually is lethal in hemizygous males. A second condition, Ehlers-Danlos syndrome variant form of periventricular heterotopia, is characterized by joint hypermobility, aortic dilatation, and nodular brain heterotopias (OMIM 300537) [Sheen et al., 2005]. Otopalatodigital (OPD) spectrum disorders are due to gain-of-function mutations of FLNA [Clark et al., 2009]. OPD spectrum disorders include otopalatodigital syndrome 1 (OPD1; OMIM 311300), otopalatodigital syndrome 2 (OPD2; OMIM 304120), Melnick–Needles syndrome (MNS; OMIM 309350), frontometaphyseal dysplasia (FMD; OMIM 305620), and terminal osseous dysplasia (TOD; OMIM 300244). OPD1 is characterized by skeletal anomalies, cleft palate, and hearing loss [Dudding et al., 1967]. OPD2 has cleft palate, micrognathia, a more severe skeletal dysplasia than OPD1, and a variety of extra skeletal anomalies [Fitch et al., 1983]. FMD is characterized by marked supraorbital ridging, deafness, digital anomalies, and tracheobronchial, cardiac, and urogenital defects [Gorlin and Cohen, 1969; Robertson et al., 2006]. MNS is defined by a skeletal dysplasia, characteristic facies, deafness, and hydronephrosis; there is usually early lethality in males [Melnick and Needles, 1966]. Affected individuals with TOD have skeletal dysplasia of the limbs, recurrent fibromas of the digits, and pigmentary defects of the skin [Bacino et al., 2000]. FLNA mutations have also been found to be pathogenic in FG syndrome 2 (OMIM 300321) [Unger et al., 2007], cardiac valvular dysplasia (OMIM 314400) [Kyndt et al., 2007], and intestinal pseudo-obstruction/congenital short bowel syndrome (OMIM 300048) [Gargiulo et al., 2007; van der Werf et al., 2013].
MATERIALS AND METHODS
Approval
The study was approved by the Institutional Review Board of Indiana University (Study # 1108006411). All participants or their legal guardian provided written informed consent.
Subjects
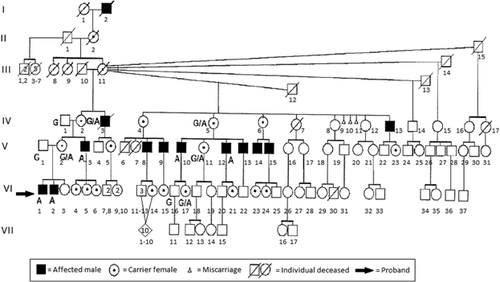
An updated seven generation pedigree of the family described by Heyen et al. [2008] has 13 affected males, 11 of whom are living (Fig. 1). Fourteen members of this Caucasian family were evaluated in the present study. These included five affected males, three obligate carrier females, two likely carrier females, one unaffected female, one unaffected male, and two unaffected husbands of obligate carrier females. Each study participant completed a medical and family history questionnaire, answered a review of systems form, and underwent a physical examination. Venipuncture was performed on all but one individual, and these blood samples were sent to the Greenwood Genetic Center for linkage, X-inactivation studies, and molecular analyses.
Linkage Analysis
Genomic DNA of members of family K7259 was prepared from fresh blood using standard protocols. Linkage analysis was conducted using 19 microsatellite markers spaced along the X chromosome.
X-Inactivation Studies
X-inactivation studies were done by standard methodology.
Chromosome X-Exome Sequencing
Illumina sequencing libraries for two affected males were made using TruSeq DNA sample preparation kits. The X chromosome-exome was enriched using an Agilent SureSelect X chromosome exome kit. The Illumina HiSeq2000 platform was used to generate the genomic sequence reads of 75–100 bp. INDEL realignment and base recalibration were conducted using GATK. The Unified Genotyper (GATK) was used for variant calling and the final variant output was pre-processed by removing variants with zero coverage from one strand and variants in close proximity (<10 bp) to another variant in the same sample.
Potential pathogenic variants were identified if they were present in both males sequenced. Variants shared with 162 unrelated male samples used in the 1000 Genomes Project were removed. Last, only those remaining variants that were in the linkage region were considered for further analysis.
RESULTS
Subjects
The key clinical features of affected males and carrier females are presented in Table I.
| Patient | Joint contractures | Keloids | Increased optic cup-to- disc ratio | Uric acid renal stones | Hypoplastic flexion creases | Fifth finger clinodactyly | Decreased joint range of motion | Prominent supraorbital ridges | Heart defect | Anxiety | Osteoporosis | Arthritis | X-inactivation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Affected males | |||||||||||||
| V-3 | X | X | X | X | X | X | X | X | X | ||||
| V-10 | X | X | X | X | X | X | |||||||
| V-12 | X | X | X | X | X | X | X | ||||||
| VI-1 | X | X | X | X | X | X | X | ||||||
| VI-2 | X | X | X | X | X | ||||||||
| Carrier females | |||||||||||||
| IV-2 | X | X | X | X | X | X | X | 75:25 | |||||
| IV-5 | X | X | X | X | 72:28 | ||||||||
| V-2 | X | X | X | 66:34 | |||||||||
| V-11 | X | X | X | X | X | 94:6 | |||||||
| VI-17 | X | 82:18 | |||||||||||
- X, feature present; blank space, feature not present or not known.
Individual VI-1
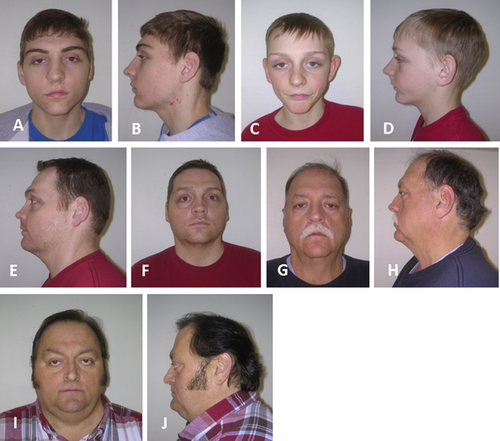
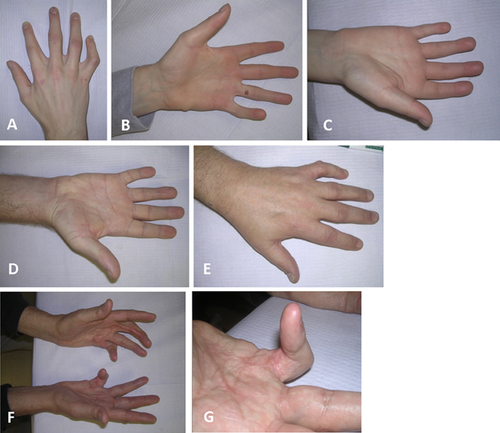
The proband is a 17-year-old male (Fig. 2A,B) who originally had been seen at 12 years of age for mild dysmorphic features, congenital DIP joint contractures, progressive stiffness of the shoulders and neck, and a large optic cup-to-disc ratio. He has had no surgeries or hospitalizations. He does have a history of a learning disability and a heart murmur. The physical examination was significant for a short forehead, mild supraorbital ridges, down slanting palpebral fissures, slightly prominent ears, a high arched palate, and extensive acne over the neck, shoulders, and back with some keloid formation. The upper extremities showed limited supination (135° on left and 145° on right), limited elbow extension of 160° bilaterally, limited extension of all fingers, slight radial deviation of the fifth fingers, hypoplastic distal flexion creases on the right third finger and left third through fifth fingers, and absent distal flexion creases on the right fourth and fifth fingers (Fig. 3A,B). His height was 183 cm (75–90th centile) and weight 72.3 kg (50–75th centile). FLNA genotype was hemizygous for a c.4726G>A mutation of FLNA.
Individual VI-2
Individual VI-2 (Fig. 2C,D) is the affected 12-year-old brother of the proband. Myringotomy tubes were placed twice due to recurrent otitis media and he underwent a hydrocele repair. He also was hospitalized at 14 months of age due to a viral GI infection and had a heart murmur that resolved on its own. Like his brother, he has an increased optic cup-to-disc ratio. He also reported that his knees “give out” when he is very active. On physical examination, his height was 155 cm (50–75th centile) and weight was 42.7 kg (50th centile). He was gaunt looking and had mildly down slanting palpebral fissures; no prominence of the supraorbital bones; slightly prominent ears; pectus carinatum; clinodactyly of the fifth fingers; contractures of third through fifth fingers bilaterally; limited range of motion of elbows, shoulders, and fingers; hypoplastic distal flexion creases of the right fourth and fifth fingers; and an almost absent distal flexion crease on the left fourth finger (Fig. 3C). He also was hemizygous for the c.4726G>A mutation in FLNA.
Individual VI-3
Individual VI-3 is the 9-year-old sister of the proband. Medical history is significant for allergies, bilateral myringotomy tube placement, and learning disability. Her height was 138.5 cm (75–90th centile) and weight was 36.4 kg (75–90th centile). Physical examination showed a high forehead, crowding of the lower teeth, and hyperextension of the elbows. FLNA genotyping was not done.
Individual VI-16
Individual VI-16 is the unaffected 32-year-old male maternal half second cousin of the proband. He underwent a tonsillectomy at age 18 and had a bone spur due to a wrestling injury removed from his nose. He also broke his left fifth finger and suffered a vertebral fracture. Reportedly, he is missing two lumbar vertebrae. He has a history of loud snoring and polydipsia. His height was 186 cm (90th centile) and weight was 118.2 kg (>95th centile). Physical examination showed left fifth finger contracture at the proximal interphalangeal (PIP) joint. He was hemizygous for the normal FLNA allele.
Individual VI-17
Individual VI-17 is a 25-year-old female and a maternal half second cousin of the proband. She is a presumed carrier of the family's FLNA mutation. She was born with an “underdeveloped esophagus,” has had irritable bowel syndrome and underwent wisdom teeth removal at age 16. She also has an increased optic cup-to-disc ratio. In addition, she reported that she suffers from headaches, allergies, and premenstrual symptoms. Her height was 168 cm (75th centile) and weight was 72.3 kg (75–90th centile). In addition, she had clinodactyly of both third fingers but otherwise had a normal physical examination. FLNA genotyping showed that she was heterozygous for the familial FLNA mutation.
Individual V-1
Individual V-1 is the 40-year-old unaffected father of the proband. He has no medical problems. On physical examination, his height was 188 cm (95th centile) and weight was 83.2 kg (75–90th centile). He had a narrow face, slightly prominent ears, and 10° of scoliosis. Testing found him to be hemizygous for the normal FLNA allele.
Individual V-2
Individual V-2 is the 37-year-old mother of the proband. She is an obligate carrier of the familial FLNA mutation. She has a history of allergies and acid reflux, and underwent esophageal dilation for a stricture. By prior exam, she also has an increased optic cup-to-disc ratio. Her height was 164.5 cm (90th centile) and weight was 89.6 kg (>95th centile). Physical examination was significant for prominent supraorbital ridges, down slanting palpebral fissures, abdominal striae, increased moles on the trunk, and mild contractures of the fifth fingers. She is a carrier of the family's FLNA mutation.
Individual V-3
Individual V-3 (Fig. 2E,F) is an affected 35-year-old uncle of the proband. He has a history of increased optic cup-to-disc ratio, extensive keloids, multiple uric acid kidney stones, and arthritis. A motorcycle accident resulted in a leg fracture that required a skin graft. Physical examination showed a height of 185 cm (75–90th centile) and weight of 108.2 kg (>95th centile). He had a narrow forehead; prominent supraorbital ridges; minimal enophthalmos; down slanting palpebral fissures, high arched palate; extensive keloids of his chest, back, right antecubital fossa, posterior thighs, and lower legs; multiple pigmented nevi on his abdomen; bilateral fifth finger clinodactyly; no distal flexion creases on the fourth digits and only hypoplastic distal flexion creases on both third and right second digits (Fig. 3D). He had minimal motion of the third and fourth DIP joints of the left hand. There was decreased flexion of both second and right third DIP joints. Further, he was unable to flex his right fourth finger and had decreased supination of both arms. Genotyping showed that he is hemizygous for the family's FLNA mutation.
Individual V-10
Individual V-10 (Fig. 2G,H) is the affected 53-year-old male maternal half-cousin once removed from the proband. Past medical history is significant for fatty liver disease, osteoarthritis, Dupuytren contractures that required surgery, uric acid kidney stones, and hypertrophic scarring after surgery. He had a keloid on his right shoulder that has reportedly resolved. He also has a history of loud snoring and anxiety. His height was 189 cm (95th centile) and weight was 123.2 kg (>95th centile). Physical examination showed prominent supraorbital ridges, down slanting palpebral fissures, scarring of the right tympanic membrane, fifth finger clinodactyly, contractures of both fifth and the left third and fourth fingers, and hypertrophic scarring of surgical incisions of the hands (Fig. 3E). Also there were Dupuytren pads of the right second and third, and both fourth fingers. This person also is hemizygous for the FLNA mutation found in the family.
Individual V-11
Individual V-11 is the 52-year-old female maternal half-cousin once removed from the proband. She is an affected carrier of the family's condition. She suffers from joint pain of her hands, wrists and hips, has sleep apnea, and at night requires continuous positive airway pressure (CPAP). Recently, she has had unexplained weight gain and fatigue. She also reports poor wound healing, blurry vision, concern for glaucoma, palpitations, loud snoring, dyspnea, back pain, left sciatic pain, osteoporosis, joint stiffness, temperature sensitivity, easy bruising, poor circulation, dizziness, numbness, anxiety, and hot flashes. A dilation and curettage and hysterectomy were performed at age 29 due to scar tissue. At age 45, she developed contractures of the hands, had a cholecystectomy at age 50, underwent a foot surgery to remove a “nerve tumor” at age 50 and had umbilical hernia repairs at ages 44 and 55 years. Physical examination showed a height of 165 cm (90th centile) and weight of 121.4 kg (>95th centile). She had a low frontal hairline, narrow ears, an increased gap between her lower central incisors, obesity, and a 3.5 cm keloid on her abdomen. There also were mild contractures of the fifth fingers and a bony/cartilage bump over the fifth finger PIP joint. Genotyping showed that she is a heterozygous carrier of the familial FLNA mutation.
Individual V-12
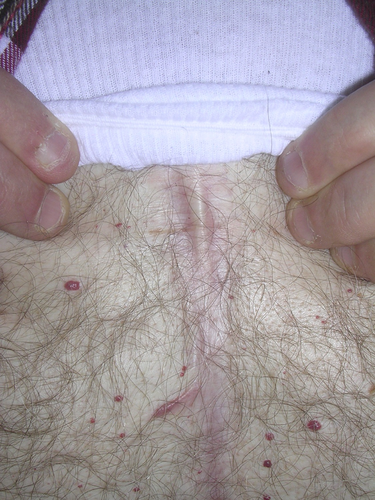
Individual V-12 (Fig. 2I,J) is an affected 51-year-old male maternal half-cousin once removed from the proband. His extensive medical history includes uric acid kidney stones, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease (COPD), congestive heart failure, atrial fibrillation, atrial septal defect (ASD), tricuspid valve stenosis, chronic bronchitis, an Escherichia coli kidney infection and right leg deep-vein thromboses. Past surgeries include a tricuspid valve stenosis and ASD repairs, appendectomy, cataract removal, and vein stripping of the right leg. He also reported palpitations, keloids, loud snoring, sleep problems, heartburn/reflux, muscle/joint pain, joint stiffness, poor circulation, allergies, and discoloration of the lower legs. His height was 175 cm (25–50th centile) and weight was 127.3 kg (>95th centile). Physical examination was significant for prominent supraorbital ridges, down slanting palpebral fissures, a geographic tongue, limited flexion of the metacarpophalangeal (MCP) joints, contracture of the left fifth finger (Fig. 3F,G), keloids on his chest (Fig. 4), a well healed scar on his left shoulder, and crepitus of the knees. He was found to be hemizygous for the FLNA mutation in the family.
Individual IV-1
Individual IV-1 is the unaffected 61-year-old maternal grandfather of the proband. Medical diagnoses include arthritis, diabetes, gastroesophageal reflux, hypercholesterolemia, and a fracture of the left fifth finger. He underwent open heart surgery at 55 years of age. He reported a “congenital deteriorated vertebral disc” that has resulted in back pain. His physical examination showed a height of 188 cm (95th centile), weight of 110.9 kg (>95th centile) and clinodactyly of the left fifth finger. He is hemizygous for the normal FLNA allele.
Individual IV-2
Individual IV-2 is the maternal grandmother of the proband. She is an obligate carrier for the familial FLNA mutation. Her extensive medical history includes keloid formation, osteoporosis, Raynaud disease, hypothyroidism with a goiter and nodules, gastroesophageal reflux, L4 and L5 degenerative disc disease, anxiety, irritable bowel syndrome, diverticulitis, melanoma, possible stroke with aphasia and possible arteriosclerosis. She has had a tonsillectomy, thyroidectomy, right breast cyst removal, uterine dilation and curettage, bilateral carpal tunnel surgery, and appendectomy. She also has undergone cortisone injections for her keloids. Additionally, she stated having dysphagia, hoarseness, vision changes, palpitations, dyspnea, constipation, polyuria, kidney stones, back and joint pain, joint stiffness, cold sensitivity, poor circulation, memory loss and lack of concentration, numbness, allergies, and hot flashes. She also has an increased optic cup-to-disc ratio. Her height was 164 cm (50th centile) and weight was 73.6 kg (75–90th centile). Physical examination was significant for midface hypoplasia, deviation of the nose to the left, webbing of the uvula, a long chin, mild limitation of flexion and hypoplastic DIP creases on the right third and both fourth fingers, limited MCP flexion on the right, keloids on the right shoulder, café au lait spot on right lower back, hypopigmentation of the abdomen, and a well healed scar on the throat. She is a heterozygous carrier of the familial FLNA mutation.
Individual IV-5
Individual IV-5 is the 70-year-old maternal half great aunt of the proband. She is an obligate carrier of the family's mutation. Her medical problems include rheumatoid arthritis, fibromyalgia, osteoarthritis, and osteoporosis. She also had a hysterectomy at 38 years of age, right wrist surgery to remove a bone spur at 43 years of age, cholecystectomy at age 46 years, foot and toe surgery at age 59, spinal surgery for 13 fractures at age 60, right rotator cuff surgery at age 62, and repair of left wrist fracture at age 65. She reported dyspnea, heartburn/reflux, polyuria, urinary incontinence, diffuse joint pain and stiffness, temperature sensitivity, easy bruising, poor circulation, numbness/tingling of hands and feet, allergies, frequent infections, and anxiety. Her height was 148 cm (<5th centile) and weight was 64.1 kg (25th centile). Significant physical examination findings included clinodactyly of the fifth fingers; enlarged MCP joints; crepitus of the elbows, shoulders, and knees; a cartilage or bony bump of the bilateral wrists and a well healed pearly flat scar on the mid chest area. Genotyping proved that she is heterozygous for the familial FLNA mutation.
Linkage Analysis
Utilization of 19 microsatellites distributed along the X chromosome allowed us to localize the candidate gene in the family reported here (K7259) to Xq27-qter, with a LOD score of 2.1.
X-Inactivation Studies
The results of the X-inactivation studies are listed in Table I.
Chromosome X-Exome Sequencing
X chromosome-exome sequencing resulted in the identification of a variant in the FLNA gene as the sole potentially relevant alteration in the linkage region. Not surprisingly, the mutation segregated in the family in affected males and obligate carrier females (Fig. 1). The variant, c.4726G>A, p.G1576R, was determined to be likely pathogenic based on various bioinformatic analyses (Table II) as well as the fact it was not present in either the Exome Variant Server or ExAC databases. Unfortunately, there does not exist a good in vitro functional assay to provide “confirmation” of the pathogenicity of the p.G1576R alteration.
| Software | Prediction |
|---|---|
| Polyphen | Probably damaging (score 1.00) |
| Sift | Damaging |
| Mutation taster | Disease causing (probability 0.99) |
DISCUSSION
We further evaluated a previously reported family [Heyen et al., 2008] with an apparently undescribed X-linked condition consisting of joint contractures, keloids, an increased optic cup-to-disc ratio and uric acid renal stones. Not unexpectedly, we again noted the previously described joint contractures, keloids, hypoplastic flexion creases, and limited range of motion of various joints reported by Heyen et al. [2008], but also noted fifth finger clinodactyly in several affected males (Fig. 1 V-3, V-10, VI-1, and VI-2) and prominent supraorbital ridges in four involved males (V-3, V-10, V-12, and VI-1) that may expand the previously described phenotype (Table I). Carrier females exhibit variable milder features including keloids (IV-2 and V-11), joint contractures (V-2 and V-11), hypoplastic flexion creases (IV-2), fifth finger clinodactyly (IV-5), and an increased optic cup-to-disc ratio (IV-2, V-2, and VI-17). Three females (IV-2, IV-5, and V-11) also report anxiety, osteoporosis, and arthritis (Table I). Linkage analysis localized the putative gene to Xq27-qter and chromosome X-exome sequencing revealed a mutation in exon 28 of the FLNA gene (c.4726G>A; p.G1576R). This mutation was seen in all tested affected males, all tested obligate carrier females and two likely carrier females. No unaffected individuals carried this mutation. Bioinformatic analyses (Table II) indicate that the p.G1576R mutation in the FLNA gene is very likely pathogenic in this family.
The c.4726G>A mutation in exon 28 of the FLNA predicts that a glycine has been replaced by an arginine at amino acid 1576 (p.G1576R). This is a novel mutation in the FLNA gene. Exon 28 encodes part of the C-terminus of repeat 13 and N-terminus of repeat 14 of filamin A [Foley et al., 2010]. The cytosolic domain of the protease furin binds to repeats 13 and 14, the extracellular calcium sensing receptor binds to repeats 14–16 (amino acids 1566–1875) and the focal adhesion-associated protein FAP52 binds to repeats 13–16 (amino acids 1524–1858) of the FLNA protein. However, we are unsure how a disruption of these processes could result in the features of affected individuals in our family [Liu et al., 1997; Awata et al., 2001; Nikki et al., 2002]. Mutations of repeats 13 and 14 have been associated with other FLNA syndromes. Foley et al. [2010] reported a female with MNS due to deletion c.4738_4755 + 10del28 spanning the exon 28-intron 28 junction, likely affecting repeat 14. A truncating mutation of exon 27 involving repeat 13 was previously described by Parrini et al. [2006] in a patient with periventricular heterotopia.
Many phenotypic findings seen in our study family have been reported in other syndromes due to FLNA mutations. Fifth finger clinodactyly, limited range of motion of joints and joint contractures/camptodactyly have all been described in patients with OPD1, OPD2, FMD, and TOD [Dudding et al., 1967; Gorlin and Cohen, 1969; Fitch et al., 1983; Bacino et al., 2000]. The prominent supraorbital ridges seen in Patients V-3, V-10, V-12, and VI-1 has been described in FMD, OPD1, and MNS [Melnick and Needles, 1966; Dudding et al., 1967; Gorlin and Cohen, 1969; Robertson et al., 2006]. Other features seen in FMD include hearing loss, ocular hypertelorism, down slanting palpebral fissures, micrognathia with a pointed chin, subglottic stenosis, ulnar deviation of the hands, arachnodactyly, hypoplasia of the distal phalanx of the thumb, urethral stenosis/atresia, scoliosis, and a generalized skeletal dysplasia [Robertson et al., 2006]. Other than for the significant but relatively mild supraorbital ridges, down slanting palpebral fissures and contractures, none of the above features associated with FMD is present in the family reported here. Keloids, uric acid renal stones, and increased optic cup-to-disc ratio appear to be specific to our family's syndrome and associated with the family's novel FLNA mutation. It is possible that one or more of these latter traits is segregating independently from FLNA but this appears unlikely since no unaffected males or non-carrier females have any of these findings. We have not done further analysis to determine the mechanism causing the uric acid renal stones. MRIs have not been done on affected individuals since none of them has had seizures as seen in individuals with periventricular heterotopia [Jefferies et al., 2010]. We are currently working with the family to have affected family members screened by echocardiogram as FLNA mutations have been associated with valvulopathy [Jefferies et al., 2010]; Individual V-12 does have a history of tricuspid valve stenosis. As noted above, the family reported here has a unique constellation of features not seen in other filaminopathies. As such, this family's condition likely represents a new filaminopathy due to a c.4726G>A FLNA mutation.
ACKNOWLEDGMENTS
We express our sincere appreciation to the family for allowing us to evaluate and publish their information. We also would like to acknowledge Cindy Skinner, sample coordinator, for her assistance. CES received grant support from NINDS (NS073854) and the South Carolina Department of Disabilities and Special Needs. CES would like to dedicate this publication to the memory of Ethan Francis Schwartz, 1996–1998.