Sleep disturbance in Mowat–Wilson syndrome
Abstract
Mowat–Wilson syndrome (MWS) is a multiple congenital anomaly syndrome caused by a heterozygous mutation or deletion of the ZEB2 gene. It is characterized by a distinctive facial appearance in association with intellectual disability (ID) and variable other features including agenesis of the corpus callosum, seizures, congenital heart defects, microcephaly, short stature, hypotonia, and Hirschsprung disease. The current study investigated sleep disturbance in people with MWS. In a series of unstructured interviews focused on development and behaviors in MWS, family members frequently reported sleep disturbance, particularly early-morning waking and frequent night waking. The Sleep Disturbance Scale for Children (SDSC) was therefore administered to a sample of 35 individuals with MWS, along with the Developmental Behaviour Checklist (DBC) to measure behavioral and emotional disturbance. A high level of sleep disturbance was found in the MWS sample, with 53% scoring in the borderline range and 44% in the clinical disorder range for at least one subscale of the SDSC. Scores were highest for the Sleep-wake transition disorders subscale, with 91% of participants reaching at least the borderline disorder range. A significant positive association was found between total scores on the SDSC and the DBC Total Behaviour Problem Score. These results suggest that sleep disorders should be screened for in people with MWS, and where appropriate, referrals to sleep specialists made for management of sleep problems. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Sleep disorders are relatively common in the general population. As many as 20–30% of children show significant sleep problems [Bruni et al., 1996; Liu et al., 2005]. Subjective complaints of insomnia occur in around one-third of adults, though fewer would meet strict diagnostic criteria [Ohayon, 2002]. Sleep disorders are often accompanied by depression and anxiety [American Psychiatric Association, 2013], poor sleep is associated with poorer cognition [Fulda and Schulz, 2001] and with decreased life satisfaction over time [Paunio et al., 2009], and overly long or short sleep has been found to increase mortality [Hublin et al., 2007]. Disturbances in children's sleep have been linked to poorer sleep quality, health and mental health in their parents [Martin et al., 2007].
The reported prevalence of sleep problems in people with intellectual disability (ID) ranges from 9% to 86% [Didden and Sigafoos, 2001; Boyle et al., 2010], and may be more common in this group than in the general population [Quine, 1991; Espie, 2000; Richdale et al., 2000; Didden and Sigafoos, 2001; Stores, 2001; Van de Wouw et al., 2012]. In children with ID, the most commonly reported issues are night waking, early waking and settling problems [Wiggs and Stores, 1996; Didden et al., 2002; Didden, 2008]. Studies have noted the persistence of sleep disturbance in children [Quine, 1991] and adults [Brylewski and Wiggs, 1999] with ID. Some have suggested that the prevalence and severity of sleep problems increases with more severe ID [e.g., Didden et al., 2002; Robinson and Richdale, 2004], but contrasting findings exist [e.g., Richdale et al., 2000; Boyle et al., 2010].
Sleep disturbances in children with ID can cause significant disruption to other family members [Stores, 2001] and are associated with increases in daytime behavior problems [Quine, 1991; Brylewski and Wiggs, 1999; Richdale et al., 2000; Didden et al., 2002; Boyle et al., 2010] and increased parenting hassles [Richdale et al., 2000]. Yet, despite the high risk of sleep disturbance and its detrimental consequences, addressing sleep problems in people with ID is often overlooked, partly because family members or carers may view them as an inevitable part of the person's condition [Wiggs, 2001]. Certain genetic syndromes causing ID show a predisposition towards sleep disturbance. These include Down syndrome, Prader–Willi syndrome, Fragile X syndrome, Rett syndrome and tuberous sclerosis complex [see Stores, 2001 for a review]. In particular, individuals with Smith–Magenis syndrome have an abnormal cycle of melatonin production, resulting in a disrupted 24-hr sleep-wake cycle [see Gropman et al., 2006 for a review].
There is scant information regarding sleep in Mowat–Wilson syndrome (MWS). MWS is a multiple congenital anomaly syndrome associated with ID and a distinct facial appearance [Mowat et al., 2003; Zweier et al., 2005]. It is caused by mutations or deletions of the Zinc Finger E-box-binding homeobox two gene (ZEB2) [Wakamatsu et al., 2001; Wilson et al., 2003]. The prevalence of MWS is estimated at one per 50,000–70,000 live births [Mowat and Wilson, 2010], and it affects males and females equally [Zweier et al., 2005; Garavelli and Cerruti-Mainardi, 2007].
MWS is associated with variable medical conditions, including agenesis of the corpus callosum, seizures, congenital heart defects, microcephaly, short stature, and Hirschsprung disease [Garavelli and Cerruti-Mainardi, 2007]. Motor milestones in infancy and early childhood are generally very delayed [Garavelli and Cerruti-Mainardi, 2007]. Prior reports suggest MWS typically leads to at least moderate and more often severe intellectual disability [Mowat et al., 2003; Adam et al., 2006; Garavelli and Cerruti-Mainardi, 2007; Evans et al., 2012]. However, recently, cases with a milder phenotype resulting from missense mutations and partial loss of ZEB2 function have been reported [Yoneda et al., 2002; Zweier et al., 2006; Ghoumid et al., 2013; Wenger et al., 2014]. Evans et al. [2012] found that compared with an age and ID-matched comparison group, people with MWS showed a high rate of oral behaviors, including bruxism, an increased rate of repetitive behaviors, and an under-reaction to pain. Results suggested a happy affect and sociable demeanour in the MWS group. Yet, over 30% showed clinically significant levels of behavioral or emotional disturbance—which was not significantly different from the age and ID-matched comparison group. Seizures affect approximately 80% of individuals with MWS [Garavelli and Cerruti-Mainardi, 2007], and may be more frequent during drowsiness and sleep [Cordelli et al., 2013; Paz et al., 2015]. Adlington et al. [2006] describe a 10-year-old male with MWS with seizures who showed night-settling problems and early waking. However, as far as we are aware, no one has systematically investigated sleep in MWS. The aim of the present study was, therefore, to investigate sleep disturbance in people with MWS. The hypotheses were that people with MWS would show a high level of sleep disturbance, and that sleep disturbance would show positive associations with behavior problems.
MATERIALS AND METHODS
Participants
This work was part of a larger study on the behavioral phenotype of MWS. The MWS Study was approved by the Human Research Ethics Committees for South Eastern Sydney Illawarra Health Service—Northern Section, The University of New South Wales, and The University of Sydney. Recruitment for the larger study has been described [Evans et al., 2012]. Participants in the larger study were 71 people with MWS and their family members. Of these 71 families, 39 participated in unstructured interviews (30 face-to-face, 9 by telephone) and 35 completed a questionnaire regarding sleep (27 of whom also completed interviews). One participant was excluded due to vast differences between results on measures making it difficult to determine a level of ID. Therefore, the sleep sample comprised 34 individuals (21 male, 13 female), ranging in age from 2.8 years to 51.1 years, with a mean of 12.86 years (SD = 9.54). Five participants were classified as having moderate ID, and 29 as severe-profound ID. MWS was previously confirmed via gene testing in all but one participant. Photographs of that participant were reviewed by a clinical geneticist (DM) to confirm the diagnosis. Of those whose diagnosis was confirmed via genetic testing, six had MWS due to a frameshift mutation, seven a stop codon, four had whole gene deletions, and one each a complex mutation and a larger deletion. For 13 participants, information on the specific mechanism causing MWS was unavailable though families had been informed the diagnosis was “confirmed.” Seventy-five percent of the sleep questionnaire sample experienced seizures, with 58.8% taking anti-epileptic medication.
Measures
The Sleep Disturbance Scale for Children [SDSC; Bruni et al., 1996]
The SDSC is a parent rating scale measuring symptomatology of the most common pediatric sleep disorders. The scale yields a total score and six subscales. T-Scores above 70 indicate a clinical disorder, while T-scores between 50 and 70 are described as “borderline.” The SDSC has strong psychometric properties and was normed on a sample of 1,157 Italian children aged 6.5–15.3 years [Bruni et al., 1996].
The Developmental Behaviour Checklist [DBC; Einfeld and Tonge, 1995]
The DBC is a questionnaire measuring behavioral and emotional problems in persons with ID. In this study, both the 96-item parent-report version (DBC-P) and the 107 item version for adults over 19 years (DBC-A) were used. However, because the DBC-A was collected for only a small number of participants, DBC-A data were converted to DBC-P using the 95 items that are common to the DBC-A and DBC-P [Mohr et al., 2011]. The DBC-P yields five subscales and a Total Behaviour Problem Score (TBPS), with good psychometric properties [Einfeld and Tonge, 2002].
Procedures
Questionnaires were sent to parents or carers of people with MWS, which included demographic questions and the DBC. Some distribution of questionnaires occurred via geneticists and doctors known to have patients with MWS. For a subset of participants, the unstructured data relating to sleep were collected as part of an interview with carers. For the face-to-face interviews, a psychologist (EE) visited the family's home. The visits interviews generally lasted around 3 hr. For the nine telephone interviews, the psychologist (EE) called the participants’ parent at a pre-arranged time.
Initially, a broad interview prompt was piloted with three participants. This was: “I'd like to know what life is like for you and your family, given that [person with MWS] has Mowat–Wilson syndrome.” However, the data generated from this prompt was variable, and so it was changed to: “Because no one has researched Mowat–Wilson syndrome before, it is difficult to know what questions to ask, and it is possible to miss some important information if we only used questionnaires such as those you have completed. So, I would like to ask you to tell me about anything that you have seen [the MWS participant's name] do and that you may have wondered ‘Is this related to Mowat–Wilson syndrome, or is it just [the MWS participant's name] that does this?’ I am most interested in behaviors and development, but you can also tell me about any medical problems or other things that you think might be relevant.” This prompt was given at the start of the interviews, and any information reported by parents or carers throughout the visit was noted by the researcher. Further questions based upon the responses given by respondents, and nonverbal prompts, were used to encourage elaboration. The information offered by carers was written down by the interviewer, either verbatim or in summary.
The SDSC was later sent to the families of 53 participants with whom the researcher had direct contact and whose parents or carers spoke English fluently. Of these, 35 (66%) responded. The SDSC was completed an average of 26.6 months following the DBC questionnaire, and an average of 20 months after the unstructured interviews.
Data Analysis
One-Sample t-tests were used to compare the sample's SDSC scores with those for the norming sample published by Bruni et al. [1996]. The proportion of participants falling into the normal, borderline, and clinical disorder range was calculated for each subscale. The DBC Total score was calculated as Mean Item Scores across all items [Taffe et al., 2008], as was the Total score of the SDSC. The association between sleep disturbance and demographic variables was explored using linear regression to model SDSC scores as a function of severity of ID, age, and gender. A regression was conducted using DBC Total mean item scores as the dependent variable and age (at time of completing DBC), severity of ID, gender, and the mean item score for the SDSC Total score as an independent variable. In this way, the influence of sleep disorders on behaviors was examined, controlling for potential confounds of age, gender, and ID severity.
RESULTS
Unstructured Interview Results Relating to Sleep
- Problems of sleep patterns, particularly early waking. Seven respondents mentioned this as a current issue.
- Appearing to require less sleep than others the same age, reported as a current problem for four and a past problem for one participant.
- Night waking and requiring resettling, a current problem for five participants.
Other themes which emerged less often included night terrors (reported by two). One participant with MWS had a diagnosis of central hypoventilation in sleep. However, three respondents reported that their relative with MWS had no sleep problems.
Questionnaire Results
Table I displays results from one-sample t-tests comparing the MWS sample's mean SDSC scores with those for the norming sample published by Bruni et al. [1996]. The MWS group's mean Total SDSC score of 45.44 (SD 14.30) was significantly higher than the norming sample for the SDSC total score (Mean 35.05, SD 7.70; t = 4.24, P < 0.001) and for four subscales (Disorders of initiating and maintaining sleep, Sleep breathing disorders, Sleep-wake transition disorders, and Disorders of excessive daytime somnolence). Meanwhile, no significant difference between the MWS group and Bruni et al. [1996]'s sleep disorder sample was found for the Sleep-related breathing disorders subscale, the Sleep-wake transition disorders subscale, and for the Disorders of excessive daytime somnolence subscale. However, for both the Total score and the Disorders of initiating and maintaining sleep subscales, the mean MWS sample score was also significantly below that for Bruni et al. [1996]'s sleep disorder sample, suggesting that the MWS group lay in between the healthy controls and the sleep disorders groups. The MWS group scored significantly below Bruni et al. [1996]'s sleep disorders group for the Disorders of arousal and Sleep hyperhydrosis subscales.
| MWS Sample n = 34 | Bruni et al. [1996] control sample n = 1,157 | One sample t-test results MWS group cf. Bruni et al. [1996] control group | Bruni et al. [1996] sleep disorders sample n = 147 | One sample t-test results MWS group cf. Bruni et al. [1996] sleep disorder group | |
|---|---|---|---|---|---|
| SDSC scale name | Mean (SD) | Mean (SD) | t | Mean (SD) | t |
| Disorders of initiating and maintaining sleep | 12.56 (4.69) | 9.90 (3.11) | 3.30** | 17.96 (6.89) | −6.72*** |
| Sleep breathing disorders | 5.21 (2.41) | 3.77 (1.45) | 3.48*** | 4.84 (2.60) | 0.89, ns |
| Disorders of arousal | 3.50 (0.96) | 3.29 (0.84) | 1.28, ns | 5.07 (2.47) | −9.52*** |
| Sleep wake transition disorders | 12.68 (4.67) | 8.11 (2.41) | 5.70*** | 12.60 (4.12) | 0.10, ns |
| Disorders of excessive daytime somnolence | 8.68 (4.20) | 7.11 (2.57) | 2.17* | 9.61 (4.23) | −1.29, ns |
| Sleep hyperhydrosis | 2.82 (1.45) | 2.87 (1.69) | −0.19, ns | 4.77 (2.90) | −7.85*** |
| Total score | 45.44 (14.30) | 35.05 (7.70) | 4.24*** | 54.87 (12.49) | −3.84*** |
- *P < 0.05; ***P < 0.001; ns, non-significant.
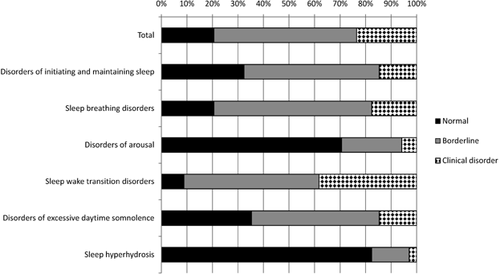
Figure 1 shows the proportion of the sample falling into the normal, borderline, and clinical disorder ranges for each subscale and the Total scale. In total, 53% fell into the borderline disorder range on at least one scale, and a further 44% fell into the “clinical disorder” category for at least one scale. Only one participant (3%) fell in the “normal” range across all scales of the SDSC. The subscale with the highest proportion of participants in the “borderline” or “clinical” disorder ranges was Sleep-wake transition disorders, with 91% reaching at least the borderline disorder range.
A linear regression was used to model the mean item score for the SDSC Total with severity of ID, age, and gender as independent variables. No significant associations were found between SDSC scores and either age, gender or severity of ID (P > 0.05 for all analyses).
Using linear regression, a significant positive association was found between the Total SDSC score DBC Total Behaviour Problem Score of the DBC, controlling for age, severity of ID, and gender (β = 0.21, 99%CI = 0.06 = 0.37; P < 0.01).
The SDSC Sleep-wake transition disorders subscale includes an item regarding sleep bruxism. Teeth grinding is associated with MWS [Evans et al., 2012]. Therefore, the sleep-wake transition disorders subscale was recalculated excluding this item and pro-rating the remaining items. Following this, the sleep-wake transition disorders scale still remained the highest in the SDSC with 56% of the sample falling into the borderline range and 29% in the clinical disorder range.
Because the age range of the study sample was broader than that of the norming group reported by Bruni et al. [1996], all analyses were repeated restricting the sample to those aged between 6.5 and 15.3 years (n = 17). All results were in the same direction as with the larger sample. Results were repeated excluding the participant for whom MWS had not been genetically confirmed. Again, results followed the same direction.
DISCUSSION
This study found sleep problems to be common in people with MWS, with all but one participant falling into the “borderline” or “clinical disorder” range for at least one scale of the SDSC. Therefore, the first hypothesis, that the MWS sample would show a high level of sleep disturbance, was partially supported. While the MWS group scored significantly higher than Bruni et al. [1996]'s control sample on the SDSC Total score, they also scored significantly lower than Bruni et al. [1996]'s sample with confirmed sleep disorders. A similar pattern was seen for the disorders of initiating sleep scale, and for two subscales (Disorders of arousal and Sleep hyperhydrosis), the MWS group did not differ from Bruni et al. [1996]'s control group. However, for the sleep breathing disorders, sleep-wake transition disorders, and disorders of excessive daytime somnolence subscales, the MWS group scored significantly higher than controls and not significantly differently than Bruni et al. [1996]'s sleep disorders sample. These findings suggest that at least some types of sleep disturbance are common in MWS.
The second hypothesis, that sleep disturbance would be associated with increased behavior problems, was supported, since an association was found between the SDSC total score and the DBC Total score. Previous findings that levels of behavior problems are similar in children with MWS compared with age- and level of ID-matched controls [Evans et al., 2012] suggest that management of behavioral issues in this group may still be required, despite findings that MWS is associated with a happy, sociable demeanour. Therefore, the current results indicate that an examination for sleep disturbance may be a useful adjunct to assessments for behavioral problems in this population. The finding that the sleep disturbance was related to behavior disturbance is consistent with previous literature showing an association sleep problems and increased daytime behavior problems in children [Quine, 1991; Wiggs and Stores, 1996; Didden et al., 2002] and adults [Brylewski and Wiggs, 1999; Boyle et al., 2010] with ID.
Sleep-wake transition disorders, which were particularly common in the MWS group, are considered parasomnias [Bruni et al., 1996]. Parasomnias are undesirable events which accompany sleep, its stages, or sleep-wake transitions [Thorpy, 2012; American Psychiatric Association, 2013]. By contrast, the types of sleep problem frequently described in the unstructured interviews were early-morning waking and frequent night waking, may reflect difficulty maintaining sleep and could be a symptom of insomnia or circadian rhythm sleep disorders [Thorpy, 2012; American Psychiatric Association, 2013]. The propensity for family carers to report problems associated with sleep patterns or amount of sleep might reflect such problems being more noticeable or more salient to parents and carers, because they disrupt the family. In contrast, the symptoms of sleep-wake transition disorders covered by the SDSC, such as twitching legs or startling movements when falling asleep, though common in the group, might present less disruption to the family and therefore, be less salient to parents when volunteering information.
The current study found no association between ID level and sleep disturbance, which is in keeping with the findings of Robinson and Richdale [2004] and Boyle et al. [2010]. However, prior literature on this topic has yielded mixed results [see Didden and Sigafoos, 2001; Van de Wouw et al., 2012 for comprehensive reviews]. One possible explanation for the current results is the high rate of sleep disturbance coupled with the small range of ID level in this sample (most participants had severe ID), along with the small sample size.
The higher rate of sleep disturbance in MWS may relate to medical conditions which often accompany the syndrome. Many conditions associated with sleep disturbance in people with ID, such as constipation, recurrent otitis media, and epilepsy [Didden and Sigafoos, 2001; Stores, 2001; Quine, 1991] are commonly seen in individuals with MWS [Adam et al., 2006]. Agenesis of the corpus callosum occurs in over 40% of individuals with MWS [Garavelli and Cerruti-Mainardi, 2007] and is associated with sleep disturbance in young children [Badaruddin et al., 2007]. Epilepsy, which is very common in MWS, shows a bi-directional association with sleep disturbance: epilepsy during sleep can induce symptoms of parasomnias, and sleep disorders or lack of sleep increase the severity of seizures and the difficulty of treating them [Grigg-Damberger, 2008]. However, a recent cohort study of adults with ID found an association between sleep problems and use of anti-epileptic drugs, but not between sleep and a diagnosis of epilepsy [Boyle et al., 2010]. The small sample size in the current study precluded examining health-related variables such as seizures, use of anti-epileptic medication, and agenesis of the corpus callosum. However, the potential for epilepsy to impact sleep in people MWS warrants further investigation, given the high rate of epilepsy and the previous findings that seizures in this group are more frequent during drowsiness and sleep [Cordelli et al., 2013; Paz et al., 2015]. Furthermore, not only might frequent epileptiform anomalies be disturbing sleep of people with MWS, but as Cordelli et al. [2013] argue, there is the potential that this impacts cognitive performance.
Implications for Management in MWS
The current findings suggest that sleep is an important issue in the management of MWS. Mowat and Wilson [2010] suggest evaluation of sleep pattern and daytime sleep for people with MWS. The current results indicate that evaluation for parasomnias and for other sleep disorders may be informative. Therefore, all types of sleep problems may usefully be screened for in patients with MWS, and referrals to sleep specialists made as appropriate. Given the high rate of epilepsy in MWS, it would be important to distinguish between parasomnias and nocturnal seizures. Lee-Chiong [2005] suggested that an expanded EEG during overnight sleep studies can aid in making such a distinction.
As Wiggs and Stores [2001] pointed out, any sleep treatment must be based upon appropriate assessment of the individual. Behavioral interventions have been shown to be successful in treating settling and/or night waking problems in children with ID [Wiggs and Stores, 1999; Montgomery et al., 2004]. Montgomery et al. [2004] found that behavioral treatments taught to parents via a booklet were just as effective as face-to-face delivery, which may prove to be a cost-effective intervention. Treatments for sleep-wake cycle disorders in people with ID include light therapy, sleep-phase retraining and melatonin [see Stores, 2002]. Such interventions may be helpful to address the early waking experienced by some children with MWS.
Limitations
The results of the study must be interpreted with several caveats in mind. The sample was small and response rate for the SDSC was relatively low (66%). Given the high proportion of participants falling into at least the borderline sleep disorder range on the SDSC, it appears unlikely that results would have differed substantially with a larger sample. Nevertheless, the possibility remains that families already affected by sleep problems were more motivated to respond.
Furthermore, sleep was assessed using a carer-report questionnaire. Direct assessment using methods such as actigraphy or polysomnography would have been preferable but this was beyond the scope of the current study. Further, it must be noted that the SDSC data was not collected at the same time as either the DBC or the unstructured interview data. Thus, although a significant association was found between the SDSC scores and the Total score of the DBC, results may not necessarily reflect contemporaneous associations between sleep and behaviors. A final limitation of the study is that the SDSC does not have norms for children with ID. Therefore, it is not possible to know from these findings whether the MWS group scored higher on sleep disturbance compared with other children with a similar level of ID. Future research could, therefore, investigate sleep in MWS using direct assessments and using instruments which include normative data for groups with ID such as that developed by Simonds and Parraga [1982], for which psychometric properties were recently established [Maas et al., 2011].
CONCLUSION
As far as we are aware, this was the first study of sleep problems in MWS. The results suggested sleep disturbance is common in people with MWS, with parents reporting problems relating to sleep patterns and early waking. The SDSC suggested a high rate of sleep-wake transition disorders. An association between sleep disturbance and behavior problems was found. These results suggest that the medical management of individuals with MWS should include screening for and treatment of sleep disorders.
ACKNOWLEDGMENTS
The authors wish to thank all the participants and their families who participated in this research. Thank you also to the clinicians who forwarded information regarding the MWS study to participants’ families, particularly Dr. Kate Gibson, Dr. Livia Garavelli, Professor Paola Cerruti-Mainardi, Dr. Wakamatsu, and Dr. Kiyokuni Miura. The MWS Study was funded by the University of New South Wales Faculty of Medicine Research Grants and the Sydney Children's Hospital Foundation. The authors thank Dr. John Taffe for his comments on an earlier version of the manuscript.




