Genotype-phenotype correlation of 16p13.3 terminal duplication and 22q13.33 deletion: Natural history of a patient and review of the literature
Abstract
This article reports a patient with a de novo ∼9.32 Mb duplication at 16p13.3 and a ∼71 Kb deletion at 22q13.33. The patient was followed from 1 month old to 3 years and 8 months of age and presented typical features of the 16p13.3 duplication syndrome. In addition, the patient presents a portal cavernoma, an alteration rarely reported in this condition. Renal agenesis was detected as additional developmental defect. After genomic array and FISH analysis, the karyotype was 46,XX,ins(22;16)(q13;p13.2p13.3). ish ins(22;16)(RP11-35P16+, RP11-27M24+). arr16p13.2p13.3(85,880-9,413,353)×3 dn arr22q13.33 (51,140,789-51,197,838)×1 dn. The authors provide a comprehensive review of the literature. This approach shed light on the genotype-phenotype correlation. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Full trisomy of chromosome 16 stands out among the autosomal abnormalities as the major cause of spontaneous miscarriage [Hassold and Jacobs, 1984]. Although rare, partial trisomy 16 p may result in a child born alive. The clinical picture is characterized by mental retardation, prenatal, and postnatal growth deficiency, facial anomalies, cleft palate, recurrent respiratory infections, seizures, congenital heart defect, and renal and genitourinary anomalies. Classical cytogenetics analysis failed to correlate the extent of the duplicated region with phenotypic severity [Martin et al., 2002].
Molecular cytogenetics techniques have enable the characterization and delineation of the critical region duplicated at 16p13.1-p13.3 [Digilio et al., 2009; Thienpont et al., 2010; Chen et al., 2012; Demeer et al., 2013].
The microduplication 16p13.3 is an emerging syndrome, characterized by a distinctive phenotype, despite differences in the location and size of duplications [Mattina et al., 2012]. Affected individuals share some major features such as characteristic facial dysmorphisms with midfacial hypoplasia in young children, and a longer face in older individuals; ocular region involvement with narrow and upslanting palpebral fissures, ptosis, and strabismus; short nose and small nostrils; and microretrognathia. Marked speech problems, mild to moderate intellectual disability, proximal implantation of thumbs, and normal growth are frequently associated features. Some patients also present mild arthrogryposis, and heart, genitalia, and palate defects [Dallapiccola et al., 2009; Chen et al., 2012; Mattina et al., 2012; Demeer et al., 2013]. Genotype-phenotype analyses of at least 26 patients with 16p13.3 duplication have been published with an expansion of the related phenotypes [Mattina et al., 2012; Demeer et al., 2013].
The 16p13.3 is a 6 Mb gene-rich region and encompasses ∼260 genes [Tüysüz et al., 2012]. Thienpont et al. [2010] firstly assessed a series of 12 patients in order to delineate the phenotypic spectrum and to perform genotype-phenotype analysis of the 16p13.3 duplication. In this study, the CREBBP was the only gene present in the critical region involved with the described phenotype. CREBBP (cyclic AMP response element (CREB)-binding protein—OMIM 600140) mutations or microdeletions (haploinsufficiency) are responsible for Rubinstein-Taybi Syndrome (RTS), which is reciprocal to the duplication 16p13.3 syndrome [Thienpont et al., 2010; Marangi et al., 2008; Demeer et al., 2013]. These findings support that human development is sensitive to CREBBP dosage [Thienpont et al., 2010; Mattina et al., 2012; Demeer et al., 2013].
The 22q13.33 region spreads ∼1,69 Mb and encompasses ∼25 OMIM genes [http://genome.ucsc.edu/]. Deletions in this genomic segment are highly variable in sizes—from 100 Kb to up 9 Mb. It has been related to Phelan-McDermid Syndrome (PMS), a contiguous gene disorder in which SHANK3 (SH3 and multiple ankyrin repeat domains 3—OMIM 606230) is the main gene involved. SHANK3 encodes a scaffolding protein in the postsynaptic densities of excitatory synapses, connecting membrane bound receptors to the actin cytoskeleton, and has been implicated in the pathogenesis and neuropathology of autism spectrum disorders (ASD). Despite the broad and heterogeneous clinical picture, the most consistent features are global developmental delay, absent or severely impaired speech, ASD, minor dysmorphic features, and hypotonia [Phelan and McDermid, 2011; Sarasua et al., 2011; Kolevzon et al., 2014].
Here, we report a patient with 9.32 Mb terminal duplication spanning from 16p13.2 to 16p13.3 and a ∼71 Kb deletion at 22q13.33 due to a nonreciprocal translocation between 16 and 22 chromosomes.
CLINICAL REPORT
The proband, a female child, was 1-month-old at her first evaluation and was referred for genetic assessment because of syndromic cleft palate. She was the third child from a healthy, non-consanguineous couple (father—40 years old/mother—27 years old) with two older brothers reported to be healthy.
Risk of miscarriage and urinary tract infection arose in the second trimester of pregnancy. There was no gestational history of epilepsy, diabetes, obesity, or substance abuse.
She was born at term but was small for gestational age: birth weight of 2.23 kg (below the 3th centile), birth length and occipital-frontal circumference 46 cm and 31 cm, respectively (both below the 3th centile). Apgar scores were eight and nine at 1st and 5th min, the amniotic fluid was meconial and she evolved with respiratory distress.
Clinical evaluation at the age of 1 month showed microcephaly (below the 3rd centile), and dysmorphic facial features with short and up-slanting palpebral fissures, blue sclera, broad nasal bridge with bulbous tip, and hypoplastic alae, thin upper lip with long philtrum, complete cleft of soft palate, mild micrognathia, and dysmorphic ears with unfolded helix and hypoplastic lobe. She had hypoplastic distal phalanges of the first and second fingers, anomalous palmar creases, broad great toes, gap between first and second toes, mild overlapping toes with and overlapping toes with proximal placement of third left toe (Fig. 1A–L).
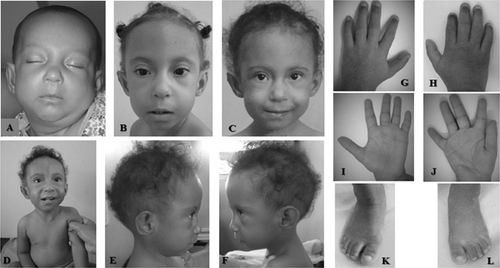
She had two episodes of upper gastrointestinal bleeding at ages of 1 and 2 years due to vascular anomalies. Digestive endoscopy showed esophageal varices associated with hypertensive gastropathy, and duodenal erosion. Follow-up at 3 months of age and 2 years and 6 months of life revealed an elongated face, sparse hair, failure to thrive, and severe psychomotor delay. Currently, at 3 years and 8 months of age, she does not walk independently, speaks unintelligible words, and follows simple instructions without autistic or other behavioral disturbances.
Ultrasonography showed atrial septal defect with mild tricuspid regurgitation, cavernous transformation of the portal vein, dilation of the splenic vein, and left kidney agenesis. Magnetic resonance imaging (MRI) of the skull and brain was normal.
MATERIALS AND METHODS
Clinical data and informed consent were obtained from the patient's parents.
Cytogenetics
Chromosome analyses by GTG banding from cultured peripheral blood lymphocytes from patient and her parents were performed at the 500 band level.
Array-GH and Molecular Cytogenetic Investigations
High resolution array was performed using the GeneChip CytoScan® HD Array Kit (Affymetrix Inc., Santa Clara, California) according to manufacturer's instructions. Data were analyzed by the Chromosome Analysis Suite (ChAS) Software (Affymetrix Inc., Santa Clara, California). Standard analysis adopted the following parameters: markers (>25 for deletion; >50 for duplication) and size (>150 kb for deletion; >300 kb for duplication). SNP-array 850K Illumina© (Illumina Inc., San Diego, California) was used to confirm results. All procedures were performed according to manufacturer's instructions.
FISH analysis was performed using two BAC clones mapped on the short arm of chromosome 16, one of them mapped within the region detected by array as duplicated (RP11-35P16) and the other located proximal of the duplicated segment (RP11-27M24).
RESULTS
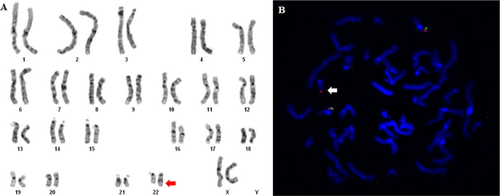
The karyotype study (GTG banding) showed an additional segment on the long arm of a chromosome 22 (Fig. 2A).
Array showed a duplication of ∼9.3 Mb spanning from 16p13.2 to 16p13.3 region (85,880–9,413,353 bp, NCBI Build 37 version [hg19]). It extends from 16p13.2 to the telomere, encompasses 190 RefSeq genes and the critical region for the 16p13.3 syndrome (http://genome.ucsc.edu/ NCBI build 37). This genomic imbalance was confirmed by a second platform (9.2 Mb deletion, 88,165–9,327,644 bp, NCBI Build 37 version [hg19]) (Fig. 3b).
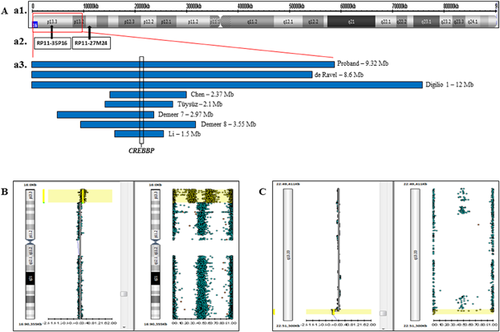
FISH analysis showed signal of the probe RP11-35P16, but no signal of the RP11-27M24 probe, on the der(22) chromosome (Figs. 3A and 2B), confirming the hypothesis that the duplicated 16p segment, as observed by array studies, was translocated to this derivative chromosome. The array study also detected a deletion of 57 Kb at chromosome region 22q13.33 (51,140,789–51,197,838 bp, NCBI Build 37 version [hg19]). Although, it was a deletion smaller than 150 kb and allele peaks results does not confirm it. This imbalance was confirmed by a different platform, presenting 71 Kb at 22q13.33 (51,140,032–51,211,392, NCBI Build 37 version [hg19]) and comprising three OMIM genes (Fig. 3c). This intersticial deletion suggests that the 16 p segment was inserted on 22qter and not simply directly translocated to the der(22).
After genomic array and FISH analysis the final karyotype was 46,XX,ins(22;16)(q13;p13.2p13.3). ish ins(22;16)(RP11-35P16+, RP11-27M24-). arr16p13.2p13.3(85,880-9,413,353)×3 dn arr22q13.33 (51,140,789–51,197,838) ×1 dn. Parents’ array and karyotype were normal. The phenotype of this patient and other published cases with 16 p duplications are shown in Table I.
| Present case | de Ravel et al., 2005 | Digilio et al., 2009a | Dallapiccola et al., 2009 | Thienpont et al., 2010b | Babameto-Laku et al., 2012 | Chen et al., 2012 | Mattina et al., 2012 | Tüysüz et al., 2012 | Demeer et al., 2013c | Li et al., 2013 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duplication size (Mb) | 9.32 | 8.6 | 12 / 8.5 | 2.5 | 0.34–2.9 | ND | 2.37 | 0.4 | 2.1 | 0.24–3.5 | 1.5 | − |
| Interstitial/Telomeric | T | T | T | I | I | T | I | I | T | I | I | − |
| Growth delay/short stature | + | − | +/− | − | 1/12 | + | − | + | + | 1/9 | + | 8/31 |
| Intelectual disability | NA | + | +/+ | + | 9/11 | + | + | + | + | 7/7 | + | 25/27 |
| RDNPM/Marked speech delay | + | + | +/+ | ND | 6/12 | + | + | + | + | 5/7 | + | 20/28 |
| High forehead | − | + | +/+ | − | 4/8 | − | + | − | − | 5/9 | − | 13/27 |
| Microcephaly | + | − | +/− | − | 0/12 | + | − | + | − | 0/9 | + | 5/31 |
| Sparse hair | + | − | −/− | − | 1/10 | − | − | − | + | 0/9 | − | 3/29 |
| Facial features | ||||||||||||
| Long face | + | − | −/− | + | 12/12 | − | − | − | + | ND | − | 15/22 |
| Blepharophimosis | + | + | +/+ | + | 5/12 | + | + | + | + | 7/9 | + | 22/31 |
| Upslanting palpebral fissures | + | − | −/− | + | 7/12 | − | + | + | + | 8/9 | − | 20/31 |
| Palpebral ptosis | − | + | +/+ | + | 3/12 | + | + | + | + | 5/9 | − | 16/31 |
| Blue sclerae | + | − | −/− | − | 0/12 | − | − | − | − | 0/9 | − | 1/31 |
| Small nares/bulbous nasal tip | + | − | −/− | + | 7/12 | − | − | + | + | 6/9 | − | 17/31 |
| Long philtrum | + | + | − /− | + | 1/12 | − | − | + | − | 0/9 | − | 5/31 |
| Thin upper lip | + | + | − /− | − | 0/12 | − | − | − | − | 0/9 | − | 2/31 |
| Micrognathia | + | + | +/+ | − | 2/12 | + | ND | + | − | 5/9 | − | 13/30 |
| Abnormalities of the ear | + | + | +/+ | − | 11/12 | + | + | + | + | 3/9 | + | 23/31 |
| Extremities | ||||||||||||
| Arthrogryposis−like features | − | − | −/+ | + | 8/12 | − | − | + | − | 4/7 | + | 16/29 |
| Proximally implanted thumbs | − | − | −/− | − | 5/12 | − | − | + | − | 7/7 | − | 13/29 |
| Nail involvement | − | − | −/+ | ND | 2/12 | − | − | − | + | 6/8 | − | 10/29 |
| Hypoplastic digital falanges | + | − | −/+ | − | ND | + | + | − | + | ND | − | 5/10 |
| Feet anomalies | + | + | −/− | + | 2/12 | − | + | + | + | ND | − | 8/22 |
| Others | ||||||||||||
| Portal cavernoma | + | − | +/− | − | 0/12 | + | − | − | − | 0/9 | − | 3/31 |
| Cleft palate/bifid úvula | + | + | −/− | − | 2/12 | + | − | − | + | 5/9 | − | 11/31 |
| Inguinal hérnia | − | − | +/− | − | 2/12 | + | − | + | − | 4/9 | + | 10/31 |
| Heart defect | + | − | +/− | − | 4/10 | − | + | − | + | 1/5 | + | 10/24 |
| Structural brain defect (MRI) | − | − | +/− | − | 3/4 | + | ND | − | + | 1/5 | + | 8/18 |
| Kidney agenesis | + | − | −/− | − | 0/12 | − | − | − | − | 0/3 | − | 1/25 |
| Genital anomalies | − | + | −/− | − | 2/12 | − | + | + | − | 0/9 | − | 5/31 |
- T, telomeric; I, interstitial; NA, not applicable; ND, no data available.
- a Two subjects.
- b Twelve subjects.
- c Nine subjects.
DISCUSSION
The patient herein described presents a rare and terminal duplication spanning from 16p13.2 to 16p13.3 (9.32 Mb) and a 71 Kb deletion at 22q13.33 due to a de novo insertional translocation. FISH analysis confirmed the insertion of the 16 p segment in the long arm of chromosome 22. According to the literature, only Kokalj-Vokac et al. [2000] described a case of insertion of 16 p into the long arm of chromosome 1, and de Ravel et al. [2005], into the short arm of chromosome 22.
The size of the duplications reported in most cases ranged from 0.24 Mb to 3.5 Mb [Thienpont et al., 2010; Demeer et al., 2013]. Previous largest duplications (12 and 8.5 Mb) were described by Digilio et al. [2009]. Duplications of ∼150 kb involving only CREBBP gene indicate that the overexpression of this gene can be the main responsible for the syndromic phenotype [Thienpont et al., 2010; Tüysüz et al., 2012; Demeer et al., 2013; Li et al., 2013]. CREBBP gene overlaps the genomic region duplicated in all 16p duplication syndrome cases reported, including our patient [Thienpont et al., 2010; Chen et al., 2012; Demeer et al., 2013]. Duplication of TRAP1 and ADCY9, flanking CREBBP, does not show dosage effect and do not affect the phenotype [Dallapiccola et al., 2009].
The present patient shows 16p13.2 duplication spanning ∼1.5 Mb and nine RefSeq genes (TMEM114, METTL22, ABAT, TMEM186, PMM2, CARHSP1, USP7, C16orf72, and MIR548X). There are no reports associating these genes and the features described in the 16p13.3 microduplication syndrome.
Our patient presents congenital heart defect and cleft palate, which are considered common findings [Digilio et al., 2009; Chen et al., 2012; Mattina et al., 2012; Tüysüz et al., 2012; Demeer et al., 2013; Li et al., 2013]. Except the patient described by Tüysüz et al. [2012] who has cleft lip and palate, all the other reports, including the present one, mention only cleft palate or bifid uvula.
Growth retardation has been reported in only two cases by Thienpont et al. [2010] and Demeer et al. [2013], while microcephaly was not described in these studies. Nevertheless, there are at least two other reports of growth retardation with microcephaly [Mattina et al., 2012; Li et al., 2013]. These features usually are associated with larger size and more telomeric extending duplications [de Ravel et al., 2005; Rochat et al., 2007; Digilio et al., 2009; Chen et al., 2012; Mattina et al., 2012; Tüysüz et al., 2012; Demeer et al., 2013; Li et al., 2013] as noted in the patient here described. Mattina et al. [2012] described the first patient with an interstitial duplication of 16p13.3 showing growth retardation and microcephaly, features typically observed in patients with duplications extending to the subtelomeric regions.
In addition, the proband has kidney agenesis, portal cavernoma, and blue sclerae. The last one is a minor sign and has not been described in this syndrome. Reports on vascular anomalies as portal cavernoma in this condition are rare and were reported by Digilio et al. [2009] in a case of 16p13.1-p13.3 duplication (∼12 Mb) and in a patient described by Babameto-Laku et al. [2012] which karyotype was 46,XY,der(4). Multiplex ligation-dependent probe amplification (MLPA) revealed duplication of the subtelomeric region of chromosome 16 p encompassing region of 16p13.1p13.3 and deletion of subtelomeric region of chromosome 4, suggesting a translocation between 4 q and 16 p.
Two largest duplications described by Digilio et al. [2009] were investigated with FISH and array-CGH and presented phenotypes overlapping features of dup16 p syndrome. It includes facial features, neurological involvement, and urinary defects. The first case presenting a duplication of region 16p13.3p13.13 with size ∼12 Mb and also 4 Mb deletion in 4q35.2 showed ectopic left kidney, and vascular abnormalities as pulmonary hypertension and portal cavernous. The second case with a duplication of 8.5 Mb in 16p13.3p13.2 and deletion of ∼300 kb in subterminal region 21q22 had vesicoureteral reflux with unilateral hydronephrosis and hypoplastic left kidney.
Other reports of renal abnormalities without genomic investigation referred to cystic transformation, hypoplasia and/or renal ectopia [Martin et al., 2002; Digilio et al., 2009; Mattina et al., 2012].
Cases with large 16 p duplications detected using standard techniques present tetralogy of Fallot or ventricular septal defect [Kokalj-Vokac et al., 2000; Martin et al., 2002; de Ravel et al., 2005; Rochat et al., 2007; Babameto-Laku et al., 2012] and Digilio et al. [2009] highlighting the association between vascular abnormalities and partial trisomy 16. Terminal hypoplasia of the hands and vascular disruption have been also reported in association with dup16 p by Martin et al. [2002]. In our patient, presence of these features and portal cavernoma suggest that vascular anomalies are related to this chromosomal imbalance.
Dallapiccola et al. [2009] and Li et al. [2013] highlighted the clinical variability of the syndrome but the reason for such variability among different sizes of microduplications is not totally understood.
Although, it was suggested that 16p13.3 duplication consists of a contiguous gene syndrome with variable phenotypic expression recently delineated at the genomic level [Dallapiccola et al., 2009], it seems that CREBBP is the critical gene responsible for the main clinical features of 16p13.3 microduplication. To date, there is no evidence of other genes in this region contributing to the phenotype. Therefore, it could be suggested that other genes play additive or modulator role justifying some of the features found in individuals with dup16p13.3.
The propositus also has a short deletion of ∼ 71 kb in 22q13.33 that encompasses two OMIM genes ACR and SHANK3. ACR is implicated in male infertility due to acrosin deficiency (OMIM). SHANK3 mutations or deletions of different sizes in 22q13.33 are related to the Phelan-McDermid Syndrome, which phenotype includes neurological deficits, mild dysmorphic features, and autism spectrum disorders [Phelan and McDermid, 2011; Sarasua et al., 2011; Kolevzon et al., 2014]. Our patient has severe developmental delay but no autistic manifestations. Kolevzon et al. [2014] also described the first case of PMS with orofacial cleft and refer 6% of renal agenesis in this syndrome.
This translocation might disrupt or delete genes located on 22q13.33 contributing to the phenotype. Conversely, ∼10% of control subjects in our in house database (10/120) carry this genomic imbalance suggesting that it may be common and benign in the Brazilian population. In fact, the present patient has not dysmorphic phenotype consistent with PMS, despite of cleft palate and renal agenesis.
This article brings the largest duplication of 16p13.3 described, along with a small 22q13.33 deletion. Renal agenesis is an additional major malformation, but its relationship with the investigated chromosomal regions could not be fully established. These features and the natural history herein reported shed light on the genotype-phenotype correlation. The application of genomic tools in other cases would contribute in this issue.
ACKNOWLEDGMENTS
The authors thank the family's cooperation. This article was supported by CAPES, CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico (# 149600/2010-0, #156674/2012-2, #473328/2013-5, #304455/2012-1), FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo (# 2011/23794-7, 2012/10071-0, 2009/00898-1, 2013/08028-1), FAPEAL—Fundação de Amparo à Pesquisa do Estado de Alagoas (#60030-700/2009).




