NBAS mutations cause a multisystem disorder involving bone, connective tissue, liver, immune system, and retina
Abstract
We report two unrelated patients with a multisystem disease involving liver, eye, immune system, connective tissue, and bone, caused by biallelic mutations in the neuroblastoma amplified sequence (NBAS) gene. Both presented as infants with recurrent episodes triggered by fever with vomiting, dehydration, and elevated transaminases. They had frequent infections, hypogammaglobulinemia, reduced natural killer cells, and the Pelger–Huët anomaly of their granulocytes. Their facial features were similar with a pointed chin and proptosis; loose skin and reduced subcutaneous fat gave them a progeroid appearance. Skeletal features included short stature, slender bones, epiphyseal dysplasia with multiple phalangeal pseudo-epiphyses, and small C1-C2 vertebrae causing cervical instability and myelopathy. Retinal dystrophy and optic atrophy were present in one patient. NBAS is a component of the synthaxin-18 complex and is involved in nonsense-mediated mRNA decay control. Putative loss-of-function mutations in NBAS are already known to cause disease in humans. A specific founder mutation has been associated with short stature, optic nerve atrophy and Pelger–Huët anomaly of granulocytes (SOPH) in the Siberian Yakut population. A more recent report associates NBAS mutations with recurrent acute liver failure in infancy in a group of patients of European descent. Our observations indicate that the phenotypic spectrum of NBAS deficiency is wider than previously known and includes skeletal, hepatic, metabolic, and immunologic aspects. Early recognition of the skeletal phenotype is important for preventive management of cervical instability. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Mutations in NBAS (neuroblastoma amplified sequence; MIM 608025) were first observed by Maksimova et al. [2010] as the cause of short stature, optic nerve atrophy, and Pelger–Huët anomaly of granulocytes (SOPH syndrome, MIM 614800). This syndrome was observed in 31 Yakut families, an isolated population of east Russia, and associated with a biallelic recessive founder mutation in NBAS (c.5741G>A, which predicts p.Arg1914His). Clinical features included postnatal growth retardation, senile face, reduced skin turgor and elasticity, osteoporosis, optic atrophy with loss of visual acuity and color vision, underlobulated neutrophils, and normal intelligence. Recently, Haack et al. [2015] reported biallelic mutations in NBAS as the cause of recurrent acute infantile liver failure in a cohort of 11 individuals from ten German families. Here, we describe the clinical phenotype of two unrelated children with NBAS mutations that were ascertained for recurrent liver failure, short stature, loose skin, and abnormal skeletal radiographs.
CLINICAL REPORTS
Patient 1 was a 12-year-old boy born to healthy Swiss parents after uneventful pregnancy and delivery (Table I). Birth weight and length were 2,360 g (3rd–5th centile) and 46 cm (3rd–5th centile), respectively. He presented at 4.5 months of life with fever, vomiting, dehydration, and elevated transaminases (up to 2,500 U/L), which normalized within 48 hr following glucose infusion. He presented several similar episodes in the first year of life, always triggered by fever. He had hypoglycemia on two occasions (plasma glucose 2.1 and 2.7 mmol/L respectively, normal >3.0), but neither cholestasis nor significant coagulopathy were observed; factor V was normal (125%, normal: 70–140%) during crises. Serology tests for a large panel of viruses did not identify an infection. At 6 months of life, hepatomegaly was noted; a liver biopsy was performed at age 8 months, showing normal histology and ruling out an inflammatory or autoimmune hepatitis; there were no signs of metabolic derangement, such as steatosis or other storage material. His hepatomegaly normalized by the age of 3 years, despite recurrent acute liver disease episodes with elevated transaminases that persisted till the age of 8 years. Early suppression of fever by mefenamic acid seemed to help in preventing hepatic crises in some instances. Blood cell count was always normal but blood smears, reviewed retrospectively, showed the Pelger–Huët anomaly of granulocytes (Fig. S1). Metabolic evaluations showed mild hyperammonemia (70 μmol/L, normal <50), normal lactate and blood gases, and urinary ketosis (beta-hydroxybutyrate 118–258 mmol/mol creatinine, normal <91.8 mmol/mol creatinine in morning urine [Boulat et al., 2003]) but inappropriately low in respect to prolonged vomiting and fasting; dicarboxylic aciduria was noted at urinary organic acids: adipate 38–119 mmol/mol creatinine (normal <11), suberate 18–26 mmol/mol creatinine (normal <6.4), 3-hydroxyadipate 23–55 mmol/mol creatinine (normal <16), 3-hydroxysebacate 93–148 mmol/mol creatinine (normal <2.7), as well as unusual presence of 3-epoxy-acid excretion (not quantified). Organic acids normalized when he was not acutely ill. Amino acid and acylcarnitine profiles in plasma and total and free carnitine were normal during and between crises. Isoelectric focusing of serum transferrin was normal. Causes of exogenous intoxication were extensively searched for and excluded. An intravenous fructose challenge excluded fructose intolerance. Very long-chain fatty acids in plasma were normal. A skin biopsy was performed and fatty acid oxidation (FAO) enzymes quantified: 3-hydroxyacyl-CoA dehydrogenase (SCHAD) activity was significantly reduced (25.2 nmol/min/mg protein; reference range 40–200) and no mutation was found in the HADH gene (MIM 601609) (data not shown). Similarly, no mutation was found in any of the FAO genes (ACSL3, MIM 602371; ACSL6, MIM 604443; ACSL5, MIM 605677; ACSL4, MIM 300157; ACSBG1, MIM 614362; ACSBG2, MIM 614363; SLC27A2, MIM 603247; ACSL1, MIM 152425; ECHS1, MIM 602292; EHHADH, MIM 607037; HSD17B10, MIM 300256; HADHB, MIM 143450; SCP2, MIM 184755; ACAA2, MIM 604770; ACAA1, MIM 604054). Because of the recurrent hypoketosis with dicarboxylic and 3-hydroxydicarboxylic aciduria during crises, and the rapid reversal of liver disease with glucose infusion, a secondary disturbance of fatty acid oxidation was suspected; fasting was limited to a maximum of 10 hr, frequent meals with slow carbohydrates were recommended and supplements of maltodextrins administered regularly, with improvement of physical endurance. With this management, the child could participate in all physical activities in social settings and at school. His learning abilities were apparently normal.
| Clinical features and laboratory abnormalities | Patient 1 | Patient 2 |
|---|---|---|
| Age | 12 | 5 |
| Nationality | Swiss | American |
| Consanguinity | – | – |
| Stature (P) | −3 SD | −3.5 SD |
| Redundant skin, reduced subcutaneous fat | + | + |
| Skeletal dysplasia | + | + |
| Cervical instability (small C1-C2) | + | + |
| Fractures | – | +a |
| Max. level of AST / ALT | 15,000/9,300 | >7,500/9,700 |
| Normal (U/L): <45/38 (12 years); <46/52 (5 years) | ||
| Hepatomegaly in infancy | + | + |
| Acute liver failure (low PT, PTT, Factor V) | – | + |
| Pelger-Huët anomaly of granulocytes | + | + |
| Hypogammaglobulinemia: normal values [Jolliff et al., 1982] | + | − |
| IgG (g/L): 6.39–13.49 (12 years); 4.63–12.36 (4–5years) | IgG 4.9–5.2 | IgG 7.39 |
| IgA (g/L): 0.7–3.12 (12 years); 0.25–1.54 (4–5 y) | IgA 0.07–0.18 | IgA 1.07 |
| IgM (g/L): 0.56–352 (12 years); 0.43–1.96 (4–5 years) | IgM 0.43–0.56 | IgM 1.61 |
| Response to vaccinations | – | n.d. |
| Reduced natural killer (NK) cells | + | + |
| Absolute NK cell count | 43 | 40 |
| Normal (cells/μl): 70–480 (12 years); 130–720 (5 years) | ||
| Frequent infections | + | + |
| Chronic/recurrent viral infections | + (VZV) | + (EBV) |
| Optic atrophy | + | – |
| Retinal dystrophy | + | – |
| Recurrent vomiting (crises) | + | + |
| Metabolic abnormalities during crises | +b | − |
| Neurological abnormalities | – | – |
| Cardiomyopathyc | – | – |
| Acute renal failurec | – | – |
| Erythema nodosumc | – | – |
| Inflammatory bowel diseasec | – | – |
| Epilepsyc | – | – |
| Celiac diseasec | – | – |
- n.d., not done/not tested.
- VZV, Varicella Zoster Virus; EBV, Epstein Barr Virus.
- a 1 skull and 1 left femur fracture.
- b Hypoketosis, dicarboxylic aciduria, and epoxy-acid excretion during crises.
- c Additional clinical features reported by Haack et al. [2015].
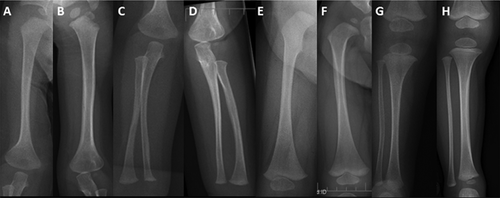
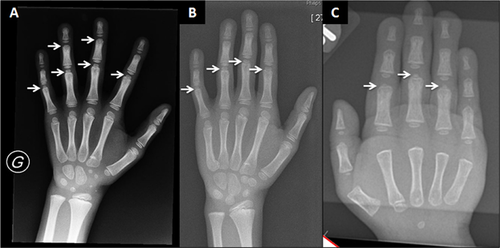
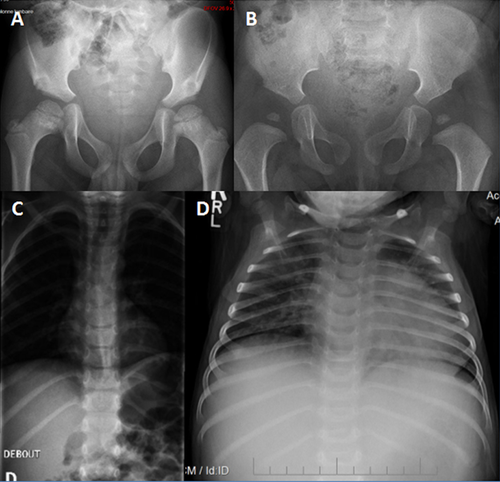
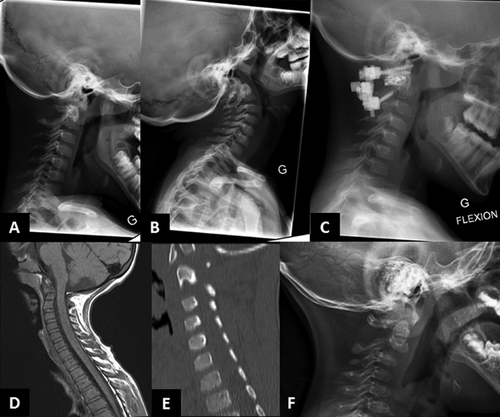
From infancy, his growth curve was slightly below the 3rd centile for weight and length; at 4.5 months his weight was 5 kg (<3rd centile), length 60.5 cm (<3rd centile), OFC 42 cm (10th–25th centile). He had a “progeroid” appearance due to redundant abdominal skin and pseudo-exophthalmos caused by reduced peri-orbital fat. During childhood, the skeletal and connective tissue phenotype became more evident, with persistent proportionate short stature (stature at 6 years was 105 cm, −2 SD), short neck, dorsal kyphosis, slender habitus with reduced subcutaneous fat at the trunk, and redundant skin (Fig. 1). Facial features (Fig. 1) included triangular face, pointed chin, proptosis, and narrow forehead. Neurologic examination was normal and psychomotor development was apparently normal. Skeletal survey (Figs. 2-4) showed long bones with mild diaphyseal stenosis but flared metaphyses, and mild epiphyseal dysplasia. At 8.5 years, there was delayed epiphyseal ossification and discordance between hand bone age (7 years) and cubital bone age (5 years) and the presence of distal pseudoepiphyses at the proximal and middle phalanges. The bones were gracile and osteopenic, despite no history of fracture. Parathyroid hormone, osteocalcin, procollagen-1-N-propeptides (P1NP), thyroid function, growth hormone and insulin secretion tests were normal. At the age of 9 years, after a fall, he presented with acute paresthesia and transient paralysis of all four limbs; X-rays and CT scan of the cervical spine showed small C1-C2 and cervical instability (Fig. 5) with compression of the cervical spinal cord, confirmed by pathologic brainstem evoked potentials. Stabilization surgery was performed with complete reversal of symptoms.
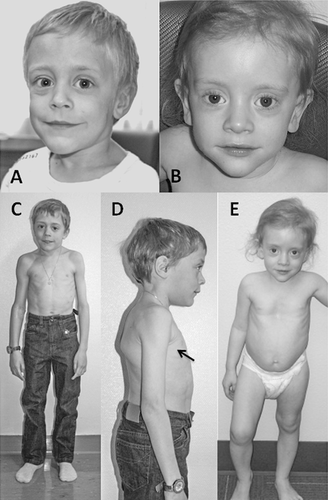
Eye examination at 5 years of age showed optic atrophy (Fig. S2) with retinal dystrophy at electroretinogram; he became symptomatic (photophobia, loss of visual acuity, and color vision) at the age of 8 years, therefore reading aids were provided.
At age 3 years, he was hospitalized for bacterial pneumonia, requiring intravenous antibiotics. He had five to eight episodes of middle ear infection per year from age 3 to 11 years, necessitating oral antibiotics. Since the age of 5 years he had recurrent labial herpes simplex virus (HSV) infection, with almost no free interval, associated with diffuse palpable lymphadenopathy. At 5 years of age, he was hospitalized for a chickenpox infection with prolonged fever, necessitating several weeks to recover; transaminases in plasma were as high as 15,000 U/L (reference values <45 U/L for AST and <38 U/L for ALT) during this infection. At age 7 years, he had a reactivation of varicella-zoster virus (VZV) infection in the thoracic region with ophthalmic involvement. A second recurrence of cutaneous and ophthalmic VZV occurred at age 11.5 years. Immunologic workup showed hypogammaglobulinemia (Table I), absent response to vaccinations, and reduced natural killer (NK) cell count (Table I). Subcutaneous weekly injections of immunoglobulins were started at age 11.5 years, restored normal IgG levels and substantially reduced both the upper airway infections and the labial HSV reactivations, with improvement of general well-being of the patient. He has not had any further acute liver crisis after 8 years of age. At the most recent evaluation (12 years), his weight was 24.7 kg (3 kg <3rd centile), stature was 131 cm (5 cm <3rd centile; −3 SD) and OFC was 54 cm (50th centile).
Patient 2, a 5-year-old girl, was born to Caucasian parents at 37 weeks of gestation with a birth weight of 2.52 kg (50th centile) (Table I). She required one week of neonatal intensive care due to transient tachypnea of the newborn. At discharge, she was able to feed normally. However, she had feeding difficulties and delayed milestones by 6 months of age. She presented at 23 months of age with fever, recurrent episodes of non-bloody, non-bilious vomiting, and elevated transaminases. Transaminases were significantly elevated (AST >7,500 U/L, normal: 16–46; ALT 9,070; normal: 33–52) which decreased during the initial crisis, but remained elevated upon discharge from the hospital. Coagulation studies were abnormal (PT 24.4 sec, normal: 11.4–15.1; PTT 39 sec, normal: 22.4–37.5, INR 2.3) and ferritin was increased (977 μg/L, normal: 10–95). She presented with hepatomegaly during the initial crisis. Liver biopsy was performed during the second crisis at 26 months of age and showed EBV nucleic acids and erythrophagocytosis suggestive of Epstein-Barr virus (EBV)-related hepatitis. Her hepatomegaly resolved after age 3 years. A metabolic evaluation during one of the crises including plasma lactate and ammonia, urine organic acids, plasma amino acids, acylcarnitine profile, succinylacetone, isoelectric focusing of transferrin, and alpha-1-antitrypsin was reported to be normal. The CSF cell counts, neurotransmitters, amino acids, and lactate were all normal. Sweat chloride testing was normal. Initial white blood cell count was low; however, all other blood counts were normal. Extensive serology for hepatotropic viruses was negative, except for chronic EBV infection. A blood smear was performed during the second acute crisis when she was 26 months and showed an absolute monocytosis. Repeat blood smear performed at 4 years showed Pelger–Huët anomaly in approximately 5% of neutrophils. An immunologic evaluation showed normal IgG and low NK cell count (Table I). In the first 2 years of life, she was hospitalized three times for acute liver failure. All crises began with fever and vomiting. Her transaminases normalized about 2 months after the prior acute crisis. Her facial phenotype included triangular face, pointed chin, down-turned corners of the mouth, and proptosis (Fig. 1). Her growth parameters were small for age; she had abundant skin and reduced subcutaneous fat (Fig. 1). During the hospitalization at 23 months of age, skeletal survey (Fig. 5) showed a J-shaped sella and small C1-C2. Cervical instability was diagnosed and fixation surgery performed. This study also showed a bone dysplasia similar to patient 1 (Figs 2-4). She has a history of two fractures (skull at 11 months and left femur at 4 years) that were the result of accidental falls. Her motor and speech development were delayed at age 3 years, but improved and motor development normalized. She still requires speech therapy for mild delays due to poor articulation. At last evaluation she was diagnosed with systemic HSV infection and her growth parameters at that time (5 years) were as follows: weight 12.3 kg (1st centile), stature 91 cm (−3.5 SD) and OFC 48.5 cm (5th centile). Eye examination at 4 years did not identify optic atrophy or retinopathy, however she did have nearsightedness with astigmatism.
Exome Sequencing (ES)
The study was conducted within the frame of a Swiss National Science Foundation-funded research project on skeletal dysplasias for which ethical approval was obtained from the State Ethic Committee (Canton Vaud). DNA was extracted according to standard protocols from leucocytes from the two probands and their parents and siblings who consented to the study. We performed ES in the DNA of patients 1 and 2 as described [Shi et al., 2011]. The results were analyzed with an informatic pipeline excluding variants present at significant frequency in public databases, and variants frequently observed in the in-house database. Results were further prioritized in terms of data quality and assuming recessive inheritance in the two probands. Candidate mutations were screened using known annotations for gene function and associated phenotypes. Sanger sequencing was used to validate the presence and status of suspected pathogenic mutations in the two probands and to confirm segregation in the parents.
Measurement of Matrix Gamma-Carboxyglutamate (Gla) Protein (MGP) Activity
Circulating MGP levels were measured in plasma from patient 1. For this study, peripheral blood was drawn in EDTA, immediately centrifuged and frozen. The MGP was measured by ELISA methods as described [Cranenburg et al., 2011].
RESULTS
ES Data
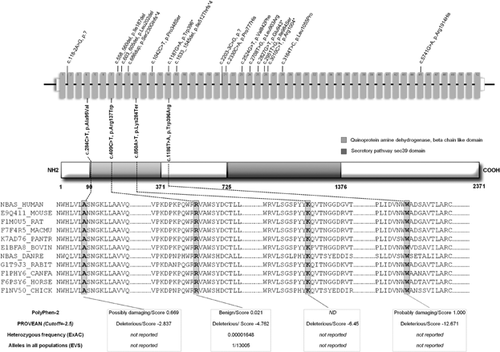
Upon inspection of the filtered ES data, biallelic mutations were found in 14 genes in common between the two unrelated probands; among them, four mutations in NBAS met the criteria of correct segregation and low frequency in control datasets: patient 1 was compound heterozygous for c.284C>T, p.Ala95Val (exon 4) and c.850A>T, p.Lys284Ter (exon 10) and patient 2 was compound heterozygous for c.409C>T, p.Arg137Trp (exon 7) and c.1186T>A, p.Trp396Arg (exon 14) (according to ENSEMBL Accession Number ENST00000281513). Three of these mutations were absent in variant databases but the missense mutation c.409C>T, p.Arg137Trp (exon 7) was listed twice in Exome Aggregation Consortium (ExAC) with a heterozygous frequency of 0.00001648 and once in Exome Variant Server (EVS). The four mutations were not found in our in-house database (>400 ES). Sanger sequencing showed the four variants were each heterozygous in one of the parents.
The PROVEAN software (Protein Variation Effect Analyzer) predicted a deleterious impact on the biological function of protein for p.Ala95Val (score −2.837; deleterious effect < −2.5), p.Arg137Trp (score −4.762) and p.Trp396Arg (score −12.671). The Polyphen-2 program v2.2.2r398 predicted the p.Ala95Val as probably damaging (score 0.982 with sensitivity 0.75 and specificity 0.96).
Figure 6 summarizes all NBAS mutations with software predictions, conservation, and population frequencies for mutations described herein.
Circulating MGP measurement in plasma of patient 1 showed normal concentration of uncarboxylated MGP: 120 pmol/L (reference values 71–756) [Cranenburg et al., 2011].
DISCUSSION
These patients presented with hepatic, metabolic, skeletal, retinal, and immunologic abnormalities that were undiagnosed until ES identified mutations in NBAS, in both. Upon review of their manifestations and other patients reported with NBAS mutations, we concluded that these variants were causative.
NBAS is a component of the syntaxin 18 complex involved in Golgi-to-endoplasmic reticulum (ER) retrograde transport of vesicles, allowing the distribution of proteins from the ER to the Golgi compartments [Aoki et al., 2009] necessary for balanced macromolecule intracellular trafficking. NBAS interacts with p31 of the syntaxin 18 complex in both HeLa cells [Aoki et al., 2009] and human fibroblasts [Haack et al., 2015]. A disturbance of retrograde Golgi-to-ER transport may result in ER stress, as suggested by expression profiling in NBAS-mutant fibroblasts [Haack et al., 2015].
NBAS is also important in nonsense-mediated mRNA decay (NMD) control. It forms an autoregulatory, conserved NMD circuit that regulates endogenous RNA targets [Longman et al., 2013]. Knockdown of NBAS causes up-regulation of genes involved in ER-stress, NMD factors, genes involved in response to wounding and nutrients, and in regulation of inflammatory response [Longman et al., 2013].
These functions suggest a pleiotropic role of this gene, potentially explaining the diversity of the phenotypic features of the patients.
Metabolic Aspects
The multisystem involvement observed in these patients resembles that of congenital disorders of glycosylation (CDG), in which defective glycosylation in the ER or Golgi affects the function of diverse proteins. However, isoelectric focusing of serum transferrin was normal in both children, thus excluding CDG type I or II and confirming the findings of Haack et al. [2015], who also found no glycosylation defect in their series.
The metabolic disturbances found in patient 1 were significant, although present exclusively during crises. The inappropriate hypoketosis and dicarboxylic aciduria during crises and the good response to glucose infusion suggested FAO dysfunction; the reduced activity of SCHAD enzyme in fibroblasts in the absence of any mutation in the HADH gene implicated a secondarily reduced HADH expression. Experiments to test this are underway. A generalized mitochondrial dysfunction as cause of the metabolic derangement in patient 1 seems unlikely because plasma lactate and urinary Krebs cycle intermediates were not elevated. Interestingly, 3-epoxy-acids were recurrently elevated in urine during crises, suggesting a peroxisomal disturbance. The NBAS protein contains a leucine zipper motif, three putative transmembrane helices, an ER signal sequence, and two peroxisomal targeting signals [Scott et al., 2003]. Our findings suggest a possible role of NBAS in regulating peroxisomal metabolism and/or protein trafficking in this organelle; further experiments are necessary to test this hypothesis.
Hepatic and Immunologic Aspects
Acute liver disease was triggered by fever in these patients and in those of Haack et al. [2015]. It has been proposed that the sensitivity to fever in these patients may be due to thermal susceptibility of the syntaxin complex 18 [Haack et al., 2015]. However, the evidence of immunodeficiency in the present patients and specific NBAS transcriptional targets identified by Longman et al. [2013] in HeLa cells suggest a link between immune dysregulation and recurrent liver inflammation. Knockdown of NBAS in HeLa cells caused upregulation of CCL20 and IL6R. CCL20 is a chemokine known to be upregulated in alcoholic hepatitis; it is a biomarker and a mediator of lipopolysaccharide-induced liver inflammation [Affo et al., 2014]. Its upregulation in NBAS deficiency may therefore facilitate liver inflammation and cytolysis upon stress factors. IL6 is a multifunctional cytokine involved in induction of immunoglobulin production from activated B-cells, in T lymphocyte proliferation, survival and differentiation, and in regulation of acute phase proteins in the liver [Zohlnhofer et al., 1992]. IL6R is expressed predominantly by hepatocytes, neutrophils, monocytes/macrophages, and some lymphocytes [Wang et al., 2013]. IL6 down-regulates IL6R via endocytosis (by internalization) and lysosomal degradation. The reappearance of the receptor at the cell surface is cycloheximide-sensitive. Degradation is likely to be protective for the cell against overstimulation [Zohlnhofer et al., 1992]. NBAS deficiency is associated with reduced degradation of IL6R mRNA in HeLa cells [Longman et al., 2013]. This may explain the hypersensitivity of liver cells to inflammatory cytokines and may provide a link between the immunologic and hepatic abnormalities in NBAS deficiency. Soluble IL6R (sIL6R) plays a role in systemic inflammatory response and its expression is induced by viral infection via the cyclo-oxygenase 2 (COX-2) pathway and inhibits viral replication [Wang et al., 2013]. This model may provide a rationale for the use of non-steroidal anti-inflammatory drugs at the onset of febrile illnesses to reduce liver inflammation in patients with NBAS deficiency.
The immunodeficiency in NBAS deficiency is subtle and possibly not consistent; patient 1 had a derangement of both humoral and cellular immune responses, and patient 2 only derangement of cellular immune response at last evaluation. Immunoglobulin replacement should be considered in affected patients to reduce frequency of infection and at the same time to avoid triggers of liver failure. Reduced NK cells may predispose to chronic viral infection and be a risk factor for tumorigenesis [Artis and Spits, 2015], for which patients should be monitored longitudinally.
Skeletal Aspects
Postnatal short stature was evident from early infancy and is a feature of the SOPH syndrome in the Yakuts [Maksimova et al., 2010]. No details on growth were provided by Haack et al. [2015], however those patients may not have been investigated because of the prominence of the hepatic involvement. Maksimova et al. [2010] suggested primary skeletal involvement in their patients, however those radiographic studies were done at adult age when dysplastic changes are usually less evident. They highlighted osteoporosis, delayed bone age, and late ossification of vertebral apophysis in one patient [Maksimova et al., 2010]. The skeletal dysplasia in the patients reported here showed thin, gracile bones, with stenotic diaphyses and flared metaphyses, and mild generalized epiphyseal dysplasia, suggesting a disturbance in bone mineralization rather than in skeletal patterning.
In the NBAS knockdown model of Longman et al. [2013], MGP was upregulated. MGP is a negative regulator of bone formation and deficiency causes Keutel syndrome, with abnormal calcification of cardiac valves, ear pinnae, and bronchial arches [Munroe et al., 1999]. Increased expression of MGP would be compatible with the bone phenotype observed in the present patients, with delayed maturation of epiphyses, thin bones, and underossified vertebrae. We measured circulating MGP in patient 1, which was in the reference range for adult controls, in contrast to upregulation found in HeLa cells [Longman et al., 2013]. Our results, although limited to one patient, do not support a role for this protein in the skeletal phenotype of NBAS deficiency.
Independent from the pathogenetic mechanism, the underossification of the cervical spine observed in these patients indicates a serious risk of neurological complications and should be assessed in patients with NBAS deficiency.
Ophthalmologic Aspects
Finally, we confirm that eye involvement includes optic atrophy and retinal dystrophy, as reported by Maksimova et al. [2010] and is not limited to the Yakut population. While optic atrophy is a frequent feature in disturbances of energy metabolism, retinal dystrophy may be linked to disturbed Golgi-to-cilium vesicle transport [Evans et al., 2010]. Therefore, NBAS is likely to play an important role in retinal homeostasis and patients should be monitored accordingly. Patient 1 had signs of a typical cone-rod dystrophy from age 5, but became symptomatic at age 8. Patient 2 did not have evidence of retinal dystrophy but her last eye examination was done at age 4. No details are available about the visual function of patients reported by Haack et al. [2015].
Our observations highlight the heterogeneous and pleiotropic clinical manifestations of NBAS deficiency. This report should raise awareness on the syndrome; we have expanded the syndrome, highlighting some clinical issues, which could be important to recognize early, such as cervical instability and immunodeficiency. Our observations also suggest possible molecular and pathogenetic mechanisms to be investigated in future research to understand and treat this disorder.
WEB RESOURCES
Lausanne Genomic Technology Facility (LGTF), University of Lausanne: http://www.unil.ch/gtf/home.htlm
UniProt, http://www.uniprot.org/uniprot/P45452
The National Heart Lung and Blood Institute (NHLBI) Exome Sequencing Project, http://evs.gs.washington.edu/EVS/
Exome Aggregation Consortium (ExAC), http://exac.broadinstitute.org/
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/index.shtml
Protein Variation Effect Analyzer (PROVEAN), http://provean.jcvi.org/index.php
SIFT, http://sift.jcvi.org/
UniProt Knowledgebase (UniProtKB), http://www.uniprot.org/
ACKNOWLEDGMENTS
The authors have no conflict of interest to declare. The study was supported by the Swiss National Science Foundation (grant 310030_132940 to L.B.). We thank the Exome Aggregation Consortium (ExAC) server for the data on mutation frequency. We thank Michael J. Bennett, Children's hospital of Philadelphia, (PA), USA, for measuring SCHAD activity and Arnold W. Strauss, University of Cincinnati (OH), USA, for HADH mutation analysis. We thank Keith Harshman of the Lausanne Genomic Technologies Facility (LGTF) at the University of Lausanne, for performing ES experiments. We thank Olivier Boulat, Clothilde Roux and Olivier Braissant of the Biomedicine laboratory at the CHUV for their contribution in the metabolic workup. We also thank Carole Chiesa for technical laboratory assistance and the patients' families for their collaboration.