New recessive truncating mutation in LTBP3 in a family with oligodontia, short stature, and mitral valve prolapse
Abstract
Latent TGFB-binding protein 3 (LTBP3) is known to increase bio-availability of TGFB. A homozygous mutation in this gene has previously been associated with oligodontia and short stature in a single family. We report on two sisters with homozygous truncating mutations in LTBP3. In addition to oligodontia and short stature, both sisters have mitral valve prolapse, suggesting a link between truncating LTBP3 mutations and mitral valve disease mediated through the TGFB pathway. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Selective tooth agenesis of one or more teeth (STHAG; OMIM 106600) is among the most common congenital disorders associated with human dentition, and affects permanent rather than deciduous dentition. Tooth agenesis may be caused by external factors, or as a part of a genetic syndrome, or as an oral-specific genetic trait. Tooth agenesis may lead to hypodontia, where one to a few teeth are missing, oligodontia, where more than six teeth are absent, excluding the 3rd molars, and anodontia, where there is absence of all teeth. Nonsyndromic sporadic or familial forms of STHAG have previously been associated with mutations in the MSX1 (STHAG1; OMIM 106600; Vastardis et al., 1996) and PAX9 (STHAG3; OMIM 604625; Stockton et al., 2000) genes, in autosomal-dominant fashion. Mutations of WNT10A are believed to cause STHAG4 (OMIM 150400) in autosomal dominant or recessive form [Van den Boogaard et al., 2012]. Mutations of EDA on Xq13.1 lead to an X-linked form of STHAG (STHAGX1: OMIM 313500; Tao et al., 2006). An additional autosomal dominant locus is suspected on 10q11.2 (STHAG5:OMIM 610926; Liu et al., 2001), and an autosomal recessive locus on chromosome 16q12.1 (STHAG2: OMIM 602639; Ahmad et al., 1998). More recently, another recessive locus, STHAG6 (OMIM 613097), was localized to 11p12–q13.3 by homozygosity-by-descent mapping in a consanguineous Pakistani family with autosomal recessive oligodontia. A homozygous nonsense variant, NM_021070.2:c.2322C > G; p.Tyr774*, in the Latent TGF-β Binding Protein 3 gene, LTBP3, was identified in affected members of this family [Noor et al., 2009]. To date, however, there have been no further reports linking LTBP3 to tooth agenesis. Here, we report on a second family in which oligodontia segregates in two girls with a truncating mutation in LTBP3, and describe the associated clinical features.
CLINICAL REPORT
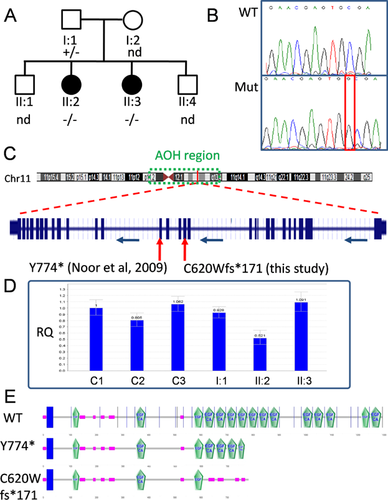
A family with oligodontia was ascertained through the Children's Hospitals and Clinics of Minnesota, and after giving written informed consent, participated in a genetics study through the Molecular Neuropsychiatry and Development lab at the Centre for Addiction and Mental Health (CAMH) in Toronto. Research ethics approval was given by the CAMH Research Ethics Board. The unaffected parents were of Emirati origin; consanguinity was not recognized. Of their four offspring, two males were unaffected and two females were affected (see Fig 1A). The father is 164 cm tall, and the mother's height is estimated to be 145 cm. Two full brothers (ages 13 and 22) have had normal growth and development. In both girls, skeletal survey showed mild narrowing throughout the mid- and posterior endplates of the cervical and lumbar vertebral bodies, focally enlarging the disc spaces; and mild scoliosis. Both patients had mild sclerosis of the vertebral endplates. Patient II:2 also had mild sclerosis of the skull base. A small pedunculated osteochondroma was also seen in the distal femur of Patient II:2. Carbohydrate-deficient transferrin profiling and N-glycan structural analysis were normal, as was 7-dehydrocholesterol level. Prothrombin time and partial thromboplastin time were normal. Qualitative and quantitative spot urine mucopolysaccharide testing was normal. Karyotype was 46,XX. SNP array showed no copy number variants, but detected a 28-Mb region of absence of heterozygosity (AOH) from 11p11.2 to 11q13.4 common to both patients. Patient II:3 had an additional 8-Mb region of AOH from 3q26.2 to 3q26.32, not present in II:2.
Patient II:2
The first patient had been born at term following an uncomplicated pregnancy. She was described as being “a little small” at birth, but birth weight is unknown. She was discharged with her mother. She had minor feeding problems for the first 10–12 weeks of life but was otherwise healthy as an infant. Her fist tooth erupted when she was 18-months old, and her teeth were described as “small and decayed.” The permanent teeth did not erupt, and crowns were placed over the deciduous teeth. In late adolescence, she had eruption of two deciduous teeth. At age 4 she was noted to have short stature. A four-month trial of growth hormone at age 13 seemed ineffective. She had menarche at age 17. She was diagnosed with mitral valve prolapse (MVP) at age 8; follow-up echocardiogram at age 18 showed MVP with mild insufficiency. Her development and learning have been normal; at 18, she was in the process of earning an accounting degree. At age 18 years 2 months, she was 136 cm tall (−4.2 SD). Body mass index (BMI) was 26.8 kg/m2. Head circumference of 53.5 cm was between the 10th and 25th centiles. She had a slightly high-pitched voice. Facial features were unremarkable. She had hypertrichosis, particularly of the lower back. She also had acanthosis nigricans of the neck and striae on the lower extremities and buttocks. She had arachnodactyly and swan necking of the fingers on extension. First metatarsals appeared short.
Patient II:3
The second patient, was also born at term following an uncomplicated pregnancy. Her birth weight was “normal.” She was discharged with her mother and had no medical problems in infancy. Her first tooth erupted at 18 months, and her teeth were described as “small and decayed.” She has had no eruption of permanent teeth and had crowns placed over her deciduous teeth. Short stature was noted when she was 4 years old. At 10 she was trialed on growth hormone without improvement in linear growth rate. She had menarche at age 13. At age 5 she was diagnosed with MVP; follow-up echocardiogram at age 15 showed MVP with mild insufficiency. Her early development was normal. She has average grades in regular classes but may have some difficulty with reading comprehension. At age 15 years 3 months, she was 132.9 cm tall (−4.6 SD). BMI was 21.3 kg/m2. Head circumference of 52.5 cm is between the 10th and 25th centiles. She had a soft, high-pitched voice. Her facial features were unremarkable. She had hypertrichosis, mild except on the back and legs, where it was more pronounced. She had swan necking of the fingers without arachnodactyly. First metatarsals appeared short.
MOLECULAR ANALYSIS
We used Sanger sequencing to screen all exons of LTBP3 in Individuals II:2 and II:3, as was described previously [Noor et al., 2009]. A single homozygous variant was identified—an insertion of “G” in exon 13, NM_021070.4:c.1858_1859insG; p.Cys620Trpfs*171 (Fig. 1B)—present in both Patients II:2 and II:3. This variant is not present in the Exome Aggregation Consortium browser of over 61,000 exomes (ExAC; Cambridge, MA; http://exac.broadinstitute.org; Oct 2014). Unaffected mother (I:2) and brothers (II:1 and II:4) were not available for screening, but the father (I:1) was heterozygous for the variant and thus the mother is an obligate carrier. The insertion leads to a frameshift, and premature truncation after 171 amino acids. Nonsense-mediate mRNA decay (NMD) was predicted (Mutation Taster: http://www.mutationtaster.org), however, analysis by qPCR on leukocyte mRNA suggests that NMD is not significant, and truncated LTBP3 protein would be generated (Fig. 1D). Analysis of RT-PCR products from the leukocyte mRNA of II:3 also showed that there were no alterations in splice site usage resulting from the mutation. The truncated protein, however, loses the majority of its functional EGF domains (only three of 15 remaining, Fig. 1E). In comparison to the Tyr774* oligodontia mutation previously reported [Noor et al., 2009], in which seven of the 15 EGF domains remain, the effect on LTBP3 function due to p.Cys620Trpfs*171 is likely to be more drastic. However, for Tyr774*, NMD seemed to be significant [Noor et al., 2009].
DISCUSSION
Because of differences in clinical evaluation and treatment between the Tyr774* family in Pakistan [Noor et al., 2009] and the p.Cys620Trpfs*171 family (this study), it is difficult to compare the clinical features and severity between the two families. However, it would appear that this family had no permanent dentition, suggesting a more severe dental phenotype than for Tyr774*. It is also worth noting that, in addition to oligodontia, short stature is a common feature and affected both this family and the Pakistani family reported by Noor et al. This feature parallels a similar phenotype see in LTBP3 null mice, which were reported to have reduced body size [Dabovic et al., 2005]. Of particular note in this family is the MVP in both affected girls. We were unable to get cardiac clinical information from members of the Pakistani Tyr774* family. Previously, TGF-β pathway activity has been shown to be upregulated in mitral valve disease [Ng et al., 2004]. Latent TGF-β Binding Proteins (LTBPs) are necessary for the targeting and activation of TGF-β, and LTBP3 in particular regulates the bioavailability of TGF-β for extracellular matrix formation in chondrocytes [Dabovic et al., 2002]. A truncating mutation in LTBP2 has previously been reported to be associated with MVP in an Iranian family [Haji-Seyed-Javadi et al., 2012]. Thus, we believe that MVP may be an important clinical feature associated with truncating mutations in LTBP3, but may not have been detected in the Tyr774* family, as cardiac phenotypes were not investigated.
ACKNOWLEDGMENTS
The authors wish to thank the patients and family for participating in this study. The authors declare that they have no conflicts of interest.