Elevation of insulin-like growth factor binding protein-2 level in Pallister–Killian syndrome: Implications for the postnatal growth retardation phenotype
Abstract
Pallister–Killian syndrome (PKS) is a multi-system developmental disorder caused by tetrasomy 12p that exhibits tissue-limited mosaicism. Probands with PKS often demonstrate a unique growth profile consisting of macrosomia at birth with deceleration of growth postnatally. We have previously demonstrated that cultured skin fibroblasts from PKS probands have significantly elevated expression of insulin-like growth factor binding protein-2 (IGFBP2). To further evaluate the role of IGFBP2 in PKS, the amount of IGFBP2 secreted from cultured skin fibroblast cell lines and serum IGFBP2 levels were measured in probands with PKS. Approximately 60% of PKS fibroblast cell lines secreted higher levels of IGFBP2 compared to control fibroblasts, although the remaining 40% of PKS samples produced comparable level of IGFBP2 to that of control fibroblasts. Serum IGFBP2 levels were also measured in PKS probands and were elevated in 40% of PKS probands. PKS probands with elevated IGFBP2 manifested with severe postnatal growth retardation. IGFBPs are the family of related proteins that bind IGFs with high affinity and are typically thought to attenuate IGF action. We suggest that elevated IGFBP2 levels might play a role in the growth retardation phenotype of PKS. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Pallister–Killian syndrome (PKS) (OMIM#601803) is a multisystem sporadic genetic diagnosis characterized by facial anomalies (prominent forehead with sparse temporal hair, broad nasal bridge, hypertelorism, wide mouth), variable developmental delay and intellectual impairment, hypotonia, seizures, pigmentary skin differences, diaphragmatic hernia, congenital heart defects, and other systemic abnormalities [Wilkens et al., 2012]. PKS is typically caused by the presence of a supernumerary isochromosome composed of the short arms of chromosome 12 resulting in tetrasomy 12p, which is often present in a tissue limited mosaic state [Peltomäki et al., 1987]. Many probands with PKS demonstrate a very unique growth pattern characterized by large birth weight and subsequent growth retardation [Wilkens et al., 2012]. The mechanism of this unique growth pattern remains unknown.
To shed light on the pathogenetic mechanism of PKS, we performed genome wide expression profiling [Kaur et al., 2014]. Insulin-like growth factor binding protein (IGFBP)-2 and -5 were identified to be significantly misexpressed with IGFBP2 expression level being 4.2 times higher in PKS skin fibroblast compared to the control fibroblast, and IGFBP5 levels about half that of controls. The expression level of other IGFBPs, as well as IGF and IGF receptor family members, were not altered.
IGFBPs consist of six family members (IGFBP-1 to IGFBP-6), that have IGF dependent and IGF independent functions [Firth and Baxter, 2002]. The IGF dependent function of IGFBPs involves the binding of free IGF proteins thereby decreasing the availability of free IGF proteins. The IGF independent functions of the IGFBPs are emerging, and several interacting proteins of IGFBP2, other than IGF, have been described [Wheatcroft and Kearney, 2009]. Given the role of IGFBPs in modulating bioavailability of IGF proteins, we hypothesized that an association exists between the abnormal IGFBP levels and the growth phenotype of PKS. In this study, we evaluate the role of IGFBP2 as a determinant of the characteristic growth phenotype seen in PKS.
METHODS
Patients
Fibroblast cell lines from a cohort of 18 probands (6 females and 12 males) with PKS and five age, gender, and race matched normal controls (2 females and 3 males) were used (Table I). The control fibroblast cell lines were ordered from the Coriell Cell Repository (coriell.org). Serum samples were obtained from 17 probands (11 females and 6 males) at the PKS family support group meeting in 2012. All of the probands were enrolled under an institutional review board approved protocol at The Children's Hospital of Philadelphia. One or more experienced clinical dysmorphologists evaluated all the affected individuals. Growth charts and physical measurements were collected from the families of these probands when available.
| Passage | Mosaic ratio % | Average mosaic ratio % | |
|---|---|---|---|
| Control 1 | P10 | ||
| P12 | |||
| Control 2 | P11 | ||
| P11 | |||
| Control 3 | P15 | ||
| P16 | |||
| Control 4 | P11 | ||
| P13 | |||
| Control 5 | P15 | ||
| P17 | |||
| Proband 1 | P10 | 0 | 0 |
| P9 | 0 | ||
| Proband 2 | P7 | 2 | 2 |
| P8 | 1 | ||
| Proband 3 | P9 | 3 | 3 |
| P10 | 2 | ||
| Proband 4 | P10 | 10 | 13 |
| P11 | 15 | ||
| Proband 5 | P6 | 30 | 23 |
| P10 | 15 | ||
| Proband 6 | P5 | 37 | 27 |
| P7 | 17 | ||
| Proband 7 | P6 | 21 | 27 |
| P8 | 33 | ||
| Proband 8 | P9 | 5 | 50 |
| P9 | 94 | ||
| Proband 9 | P9 | 53 | 50 |
| P10 | 47 | ||
| Proband 10 | P8 | 47 | 52 |
| P10 | 56 | ||
| Proband 11 | P unk+2 | 53 | 54 |
| P unk+4 | 54 | ||
| Proband 12 | P4 | 54 | 54 |
| P6 | 54 | ||
| Proband 13 | P4 | 61 | 58 |
| P6 | 55 | ||
| Proband 14 | P8 | 64 | 70 |
| P9 | 75 | ||
| Proband 15 | P4 | 70 | 75 |
| P10 | 79 | ||
| Proband 16 | P7 | 99 | 83 |
| P7 | 67 | ||
| Proband 17 | P4 | 88 | 89 |
| P9 | 89 | ||
| Proband 18 | P10 | 100 | 98 |
| P8 | 95 |
Skin Fibroblast Culture
Fibroblasts cell lines were cultured in RPMI 1,640 (Life Technologies, Carlsbad, CA) supplemented with 20% FBS (Hyclone South Logan, UT), and antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin), and 1% L-glutamine (Life Technologies). Approximately 3.5 × 105 exponentially growing cells were seeded in 5 ml of media in 25 ml FalconTM tissue culture flasks (BD Biosciences, San Jose, CA). When the flasks were 40–90% confluent, culture media was changed to serum free RPMI media, and after 48 hr, the media was collected for IGFBP2 protein measurement. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2. Cells were trypsinized (0.25% trypsin–EDTA) and split at 75–80% confluency. Cell number was counted prior to the DNA extraction. Total DNA were isolated from the same cultures for culture supernatant collection. The mosaic ratio of PKS skin fibroblast cell lines was quantified by droplet digital PCR [Izumi et al., 2014].
IGFBP2 Level Measurement
IGFBP2 levels of the culture media was measured by IGFBP2 Human ELISA Kit (ab100540) (Abcam,Cambridge MA), according to the manufacture's protocol. The culture supernatant IGFBP2 level was measured in triplicate, and normalized against the estimated total cell number in the tissue culture flask. IGFBP2 level was measured twice as biological duplicates. Collected serum samples were sent to LabCorp (Burlington, NC) for IGFBP2 measurement. Serum IGFBP2 levels were measured by radioimmunoassay (RIA).
IGFBP2 Promoter Methylation Analysis
Genomic DNA was extracted from hTERT immortalized skin fibroblast lines using NucleoSpin® tissue kit (Macherey-Nagel, Düren, Germany). Bisulfite treatment of the genomic DNA was performed with EZ DNA Methylation Gold Kit (Zymo Research, Orange, CA), following the manufacturer's instructions. The IGFBP2 promoter region was amplified by PCR using primers designed as follows: Forward, 5′- GGGAAGAGTAGGGAATTTTTAGAGTT -3′; Reverse, 5′- CCCAATAACAACAACAACAAC -3′. The amplified PCR fragments were cloned into the pGEM T-Easy vector system (Promega, Madison, WI), and 10 clones were sequenced for each sample to determine methylation status.
RESULTS
Culture Supernatant IGFBP2 Level
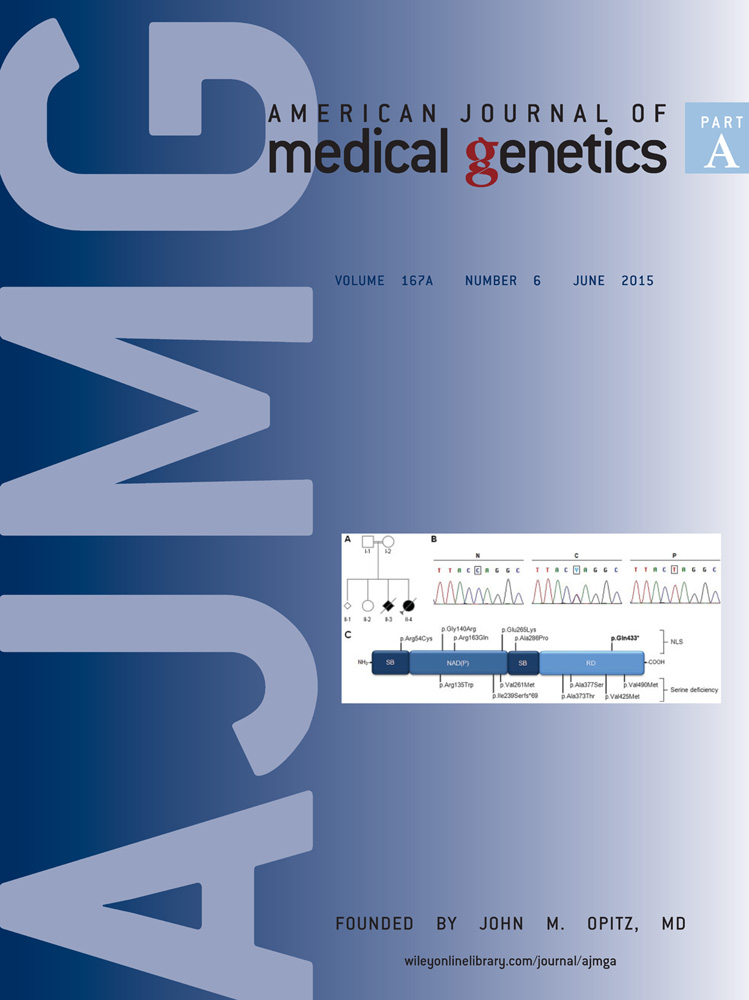
Culture supernatant samples for IGFBP2 measurements were obtained from 18 probands with PKS and 5 control fibroblast cell lines (Table I and Fig. 1a). IGFBP2 levels correlated well amongst biological duplicates using different passages except for a few PKS samples. In general, IGFBP2 levels from culture supernatant obtained from control cell lines were either undetectable or very low, while in the PKS samples, approximately 60% of the cell lines produced higher levels of IGFBP2 than controls. The remaining 40% of PKS samples produced very low IGFBP2. There was no clear correlation found between mosaic ratio and the level of IGFBP2 levels. In the samples from Probands 6, 10, and 14 two biological replicates demonstrated significantly different levels of IGFBP2. This variation may be partly explained by the different mosaic ratio between duplicates. In these three probands, the fibroblast samples with higher levels of mosaicism demonstrated higher IGFBP2 levels. In the sample from Proband 8, the two skin fibroblast cell lines used for the IGFBP2 assay had very different isochromosome 12p mosaic ratio (5% vs. 94%). However, regardless of mosaic ratio, the level of IGFBP2 in this proband was nearly undetectable.
Since our previous expression profiling demonstrated reduced IGFBP5 levels in PKS skin fibroblast compared to controls, we attempted to measure culture supernatant IGFBP5 levels, however they were undetectable.
Serum IGFBP2 Level
Serum samples were obtained from 17 probands with PKS (Fig. 1b). Seven probands had higher serum IGFBP2 levels compared to the reference range. Nine probands had normal IGFBP2 levels, and one proband had a low IGFBP2 level.
Growth Parameters
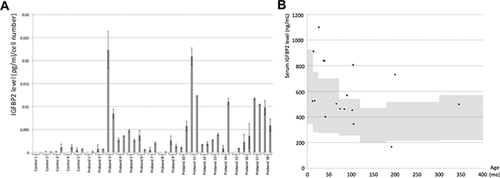
For the 17 probands in whom we were able to obtain serum samples, growth charts were available from eight (4 with high IGFBP2, 3 with normal IGFBP2, and 1 with low IGFBP2) (Fig. 2). Three out of four probands with high IGFBP2 levels (PKS13, PKS23 and PKS77) had very severe growth retardation, while the three probands with normal IGFBP2 levels demonstrated a relatively normal growth pattern. The Proband PKS76 with a low IGFBP2 level also manifested with severe growth retardation, although this proband was adopted at 11 years of age and detailed growth parameters from infancy were unavailable (data not shown).
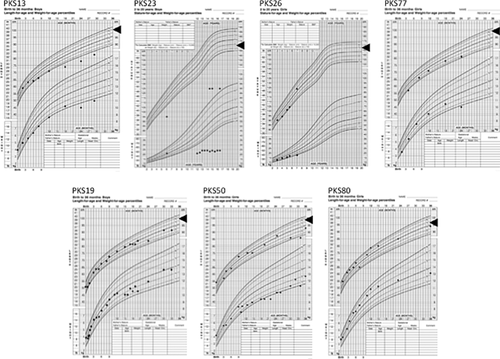
Methylation Level of IGFBP2 Promoter
Previously, hypermethylation of IGFBP2 promoter was associated with low IGFBP2 gene expression in lung cancer cells [Sato et al., 2006]. Therefore, we tested if PKS cell lines have hypomethylation of IGFBP2 promoter as a mechanism of elevated IGFBP2 level. However, the majority of IGFBP2 promoter CpG islands were unmethylated in all the skin fibroblast cell lines tested, and there were not major differences in methylation level among the PKS and control samples (Fig. 3).
DISCUSSION
PKS exhibits an unusual growth pattern characterized by prenatal overgrowth and postnatal growth failure. Here, we demonstrate that PKS skin fibroblasts secrete higher amounts of IGFBP2 in 60% of PKS probands, and serum IGFBP2 is elevated in 40% of PKS probands. Furthermore, the probands with high IGFBP2 tend to show more significant growth failure. Several IGFBP2 overexpression mouse models have been created [Hoeflich et al., 1999, 2001; Eckstein et al., 2002; Wheatcroft et al., 2007]. These mice do not show any major phenotype at birth, however, subsequently, these mice demonstrated postnatal growth retardation. This observation suggests that IGFBP2 is a negative regulator of postnatal growth [Hoeflich et al., 1999, 2001; Eckstein et al., 2002; Wheatcroft et al., 2007]. Therefore, collectively, these findings suggest a role for the elevated IGFBP2 levels in the growth retardation phenotype of PKS. However, since one PKS proband (PKS76) demonstrated severe growth failure even with a low IGFBP2 level, there are likely be other factors influencing the growth pattern in individuals with PKS.
The molecular mechanisms controlling IGFBP2 levels remain largely unknown. Elucidation of such a mechanism would be important given the fundamental role of IGFBP2 not only in somatic growth, but also in the pathogenesis of cancer and diabetes mellitus. Recently, the anti-diabetic role of IGFBP2 via modulating leptin sensitivity was described [Hedbacker et al., 2010]. It is intriguing that some patients with PKS have hypoglycemia. In our cohort of probands with PKS, at least 5 of 79 probands (6%) reported a history of hypoglycemia (data not shown). It is possible that elevated IGFBP2 levels may exert an effect to lower blood sugar levels, although hypoglycemia may be multi-factorial in etiology.
Genetic disorders associated with high IGFBP2 level provide a unique opportunity to understand the molecular mechanism regulating IGFBP2 level. Previously, in several genetic syndromes including 3M syndrome caused by OBSL1 mutations, Sotos syndrome caused by NSD1 mutations and Alstrom syndrome caused by in ALMS1 mutations, abnormalities of IGFBP2 mRNA level or plasma IGFBP2 level have been reported [De Boer et al., 2004; Maffei et al., 2007; Huber et al., 2010]. These observations suggest a role for OBSL1, NSD1, and ALMS1 in regulating IGFBP2 level. Likewise, our finding of higher IGFBP2 level in PKS suggests the presence of a gene or gene(s) on 12p controlling the expression of IGFBP2. Isochromosome 12p is often found in testicular germ cell tumors, and interestingly, serum IGFBP2 levels are elevated in patients with testicular germ cell tumor [Fottner et al., 2008]. This lends support to the presence of a critical gene(s) controlling IGFBP2 on chromosome 12p.
While in PKS the isochromosome 12p is almost always found in a mosaic state IGFBP2 can exert effects as an endocrine and/or paracrine factor and therefore over-secretion of IGFBP2 likely has an effect not only on cells that have the isochromosome 12p but also on karyotypically normal cells. Interestingly, there was no correlation between the mosaic ratio and culture supernatant IGFBP2 levels. Even with a very low mosaic ratio that was close to 0% mosaicism, some of the samples (e.g., Proband 3) secreted a very high level of IGFBP2. This observation may imply that the karyotypically normal cell population in PKS individuals also possesses the capability of secreting IGFBP2 similar to that of isochromosome 12p containing cells.
It remains unclear why a subset of PKS probands produce higher amounts of IGFBP2 protein. Similar variation of IGFBP2 mRNA level within PKS probands was found in our previous expression profiling using PKS skin fibroblast samples. Among the 18 probands, four were found to have normal IGFBP2 mRNA levels, which was comparable to control fibroblast samples. Similar to the culture supernatant IGFBP2 levels, there was no correlation between the mosaic ratio and the IGFBP2 level (unpublished observation). Another possibility we considered was a parent of origin effect of the isochromosome 12p, with activation of a gene on only the mother's or father's allele. However, no parent of origin effect was observed (data not shown). We have tested the parental origin of isochromosome 12p in two probands (8 and 17) using parental saliva DNA samples. Isochromosome 12p of Proband 8 (MAC223) and Proband 17 (PKS78) are both maternal in origin, although Proband 17 secreted very high amount of IGFBP2 and Proband 8 produced nearly undetectable levels of IGFBP2 (data not shown). Therefore, the parental origin of isochromosome 12p is unlikely to be the explanation for wide variation of IGFBP2 level in PKS probands.
The IGF axis plays an important role not only in somatic growth but also in brain development, as demonstrated by germline mutations of IGF1 and IGF1R that are occasionally associated with developmental delay, hearing loss and microcephaly [Woods et al., 1996; Walenkamp et al., 2005; Ester et al., 2009; Van Duyvenvoorde et al., 2010]. IGFBP2 is the most predominant IGFBP in CNS; therefore, we speculate that high IGFBP2 secretion may contribute to the neurological phenotype seen in PKS [Ocrant et al., 1990]. Excessive IGFBP2 may suppress cell growth in the brain, leading to microcephaly and the neurologic phenotype. In IGFBP2 overexpression mouse models a neurological phenotype was not described; however, the weight of the transgenic mouse brains was less than that of control [Hoeflich et al., 1999, 2001]. In fact, the postnatal head circumference of probands with PKS declined to lower percentiles as compared to their birth head circumference [Wilkens et al., 2012]. Therefore, it is possible that over-secretion of IGFBP2 may result in slowed brain growth as evident by slow postnatal head circumference increase.
In summary, we demonstrated that approximately half of PKS patients produce high IGFBP2 and we demonstrate the possible contribution of elevated IGFBP2 levels to the postnatal growth retardation phenotype. The clinical implication of elevated IGFBP2 level beyond the growth retardation phenotype remains unknown, however given the fundamental role of the IGF signaling pathway in cellular proliferation, elevated IGFBP2 may have a wider role in the pleiotrophic phenotype seen in PKS.
ACKNOWLEDGMENTS
We are deeply indebted to the PKS families who participated in this study as well as support from the PKS Kids family support group, The Children's Hospital of Philadelphia institutional development funds (IDK) and Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (B) Grant Number 26870139 (KI). We thank Drs. Adda Grimberg, Laura Conlin and Matthew Deardorff for their helpful suggestions on experimental design and interpretation of the data. We also thank Drs. Adam Zahm, Nicholas Hand, and Joshua Friedman for their technical assistance.