Identification of a premature stop codon mutation in the PHGDH gene in severe Neu-Laxova syndrome—evidence for phenotypic variability
Abstract
In some cases Neu-Laxova syndrome (NLS) is linked to serine deficiency due to mutations in the phosphoglycerate dehydrogenase (PHGDH) gene. We describe the prenatal and postnatal findings in a fetus with one of the most severe NLS phenotypes described so far, caused by a homozygous nonsense mutation of PHGDH. Serial ultrasound (US) and pre- and postnatal magnetic resonance imaging (MRI) evaluations were performed. Prenatally, serial US evaluations suggested symmetric growth restriction, microcephaly, hypoplasia of the cerebellar vermis, micrognathia, hydrops, shortened limbs, arthrogryposis, and talipes equinovarus. The prenatal MRI confirmed these findings prompting a diagnosis of NLS. After birth, radiological imaging did not detect any gross bone abnormalities. DNA was extracted from fetal and parental peripheral blood, all coding exons of PHGDH were PCR-amplified and subjected to Sanger sequencing. Sequencing of PHGDH identified a homozygous premature stop codon mutation (c.1297C>T; p.Gln433*) in fetal DNA, both parents (first-cousins) being heterozygotes. Based on previous associations of mutations in this gene with a milder NLS phenotype, as well as cases of serine deficiency, these observations lend further support to a genotype-phenotype correlation between the degree of PHGDH inactivation and disease severity. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Neu-Laxova syndrome (NLS) is a lethal genetic condition characterized by multiple congenital anomalies and ectodermal abnormalities [Neu et al., 1971; Laxova et al., 1972]. The diagnostic findings of NLS are characteristic and include intrauterine growth restriction with reduced fetal mobility and arthrogryposis, ichthyosis, microcephaly, short neck, and hypoplastic lungs [Manar & Asma, 2010]. Other neurologic and facial findings are suggestive of a NLS diagnosis, such as lissencephaly, cerebellar hypoplasia, corpus callosum abnormalities, severe proptosis with ectropion and hypertelorism, micrognathia, flat nose and malformed ears. Most NLS patients die shortly after birth [Manning et al., 2004]. Cerebro-oculo-facio-skeletal, Miller-Dieker, Roberts, Refsum, Pena-Shokeir and Smith-Lemli-Opitz syndromes, congenital ichthyosis and some chromosomal abnormalities (trisomy 12 and 4p deletion) should be considered in the differential diagnosis.
As it is the case for most rare genetic disorders, there are no reliable prevalence estimates for NLS; however, it appears to occur more commonly among inbred populations, and at least a dozen families with multiple gestations affected by NLS have been described [Neu et al., 1971; Laxova et al., 1972; Shved et al., 1985; Ejeckam et al., 1986; Karimi-Nejad et al., 1987; Tolmie et al., 1987; Ostrovskaya & Lazjuk, 1988; Naveed et al., 1990; Abdel Meguid & Temtamy, 1991; Rode et al., 2001; Acuna-Hidalgo et al., 2014; Shaheen et al., 2014]. Accordingly, autosomal recessive inheritance for NLS was always suspected, and recently confirmed and shown to be linked to deficiency of serine metabolism [Acuna-Hidalgo et al., 2014; Shaheen et al., 2014].
A consanguineous (first-cousin) couple was referred to our Institution during their fourth pregnancy due to the recurrence of a severe, likely autosomal recessive condition. Here, we report on the prenatal and postnatal application of magnetic resonance imaging (MRI) and complete evaluation to establish the diagnosis of NLS. A homozygous, premature stop codon mutation in the phosphoglycerate dehydrogenase (PHGDH) gene was identified in this patient, thus providing further support to the involvement of this locus in the pathogenesis of NLS, as recently suggested [Acuna-Hidalgo et al., 2014; Shaheen et al., 2014].
MATERIALS AND METHODS
Serial morphologic US evaluations were performed from 12 to 25 weeks of gestation. Upon establishment of a lethal condition, standard MRI scanning was performed. Postnatally, whole-body radiography (babygram) and an MRI were also done.
Peripheral blood samples were collected after delivery from the fetus and both parents for DNA analysis. Since the publication by Shaheen et al. [2014] identified homozygous mutations in PHGDH in three unrelated Saudi Arabian patients, the whole gene was amplified by polymerase chain reaction (PCR) in the parent-fetus trio and subjected to conventional Sanger sequencing (primers and PCR conditions available upon request). The nomenclature and position of the identified mutation is in accordance to the PHGDH reference messenger RNA sequence NM_006623.3, obtained from the National Center for Biotechnology Information. Functional prediction of the identified mutation was performed using the MutationTaster algorithm [Schwarz et al., 2010].
Informed consent for the anonymous disclosure of patient data and pictures was obtained from the family reported here.
RESULTS
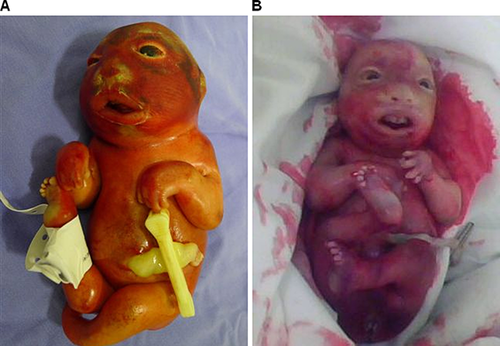
A consanguineous couple (first-degree cousins; 29-year-old mother and 43-year-old father) was referred for evaluation during their fourth pregnancy due to detection of a fetus with multiple anomalies with familial recurrence. Their first pregnancy ended in spontaneous abortion at 8–9 weeks of gestation, while the second pregnancy gave rise to a healthy daughter, who is now 8 years old. In the third gestation, the fetus presented with severe growth restriction and multiple abnormalities. Preterm labor occurred at 28 weeks of gestation and the baby died shortly after birth, but, unfortunately, the patient was not further evaluated and remained undiagnosed. Retrospectively, clinical pictures taken by the father were reviewed and compared to the prospective case, fitting the diagnosis of NLS (Fig. 1).
In the present case, an ultrasound (US) evaluation at 19 weeks of gestation detected symmetric growth restriction, hypoplasia of the cerebellar vermis, micrognathia, hydrops, possible spina bifida, shortened limbs, articular rigidity, and club feet. These abnormalities were confirmed in a subsequent US examination at 22 weeks of gestation. Supplementary Table I shows the body measurements obtained in the sequential US investigations, evidencing marked growth retardation with several data points falling below the first centile for gestational age.
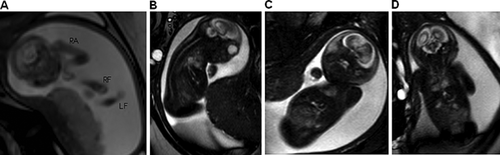
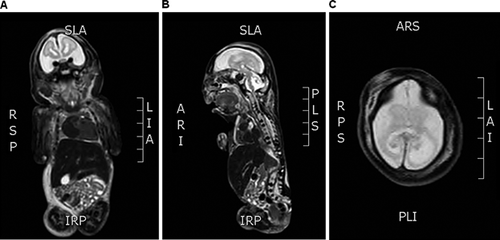
Amniocentesis showed a normal female chromosome constitution (46, XX). Considering the severity of the case, magnetic resonance imaging (MRI) was elected at 25–26 weeks of gestation in an attempt to better characterize the abnormal findings evidenced by the US evaluations (Fig. 2). It showed marked fetal growth restriction, microcephaly, a flat nose, micrognathia, talipes equinovarus, and arthrogryposis of the upper limbs. There was also a hypersignal with thickening of cranial subcutaneous tissues, but no evidence of myelomeningocele. Collectively, these findings led to the prenatal diagnostic hypothesis of NLS, confirmed postnatally.
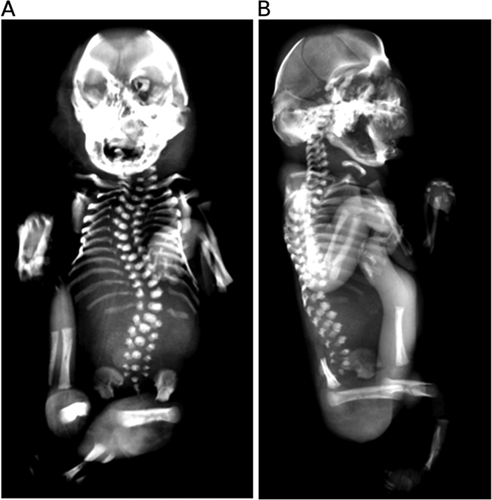
Preterm labor followed by spontaneous delivery occurred at 31–32 weeks of gestation. The stillborn infant weighted 450 g (below 1st centile), and had anthropometric measures compatible with 22–24 weeks of gestation, severe growth restriction, along with contractures of upper and lower limbs with hypoplasia of digits, “stretched” skin with mild ichthyosis and generalized subcutaneous edema, short neck, scoliosis, club feet, and female external genitalia (Fig. 1). Craniofacial findings included microcephaly, hypertelorism and malformed ears, proptosis with ectropion, depressed nasal bridge, micrognathia, and complete posterior cleft palate. An autopsy was performed and internal organ malformations consisted mainly of pulmonary, thymic, and left ventricle hypoplasia. Skeletal evaluation by radiography showed fusion of the bones of the cranial vault and scoliosis; no signs of other major malformation or dysplasia were detected (Fig. 3).
Postnatal MRI (Fig. 4) confirmed the severe involvement of the cerebellum and agenesis of the corpus callosum, as indicated by the diagnostic procedures performed in utero. Furthermore, absence of cerebral gyri and sulci reinforced the prenatal finding of lissencephaly.
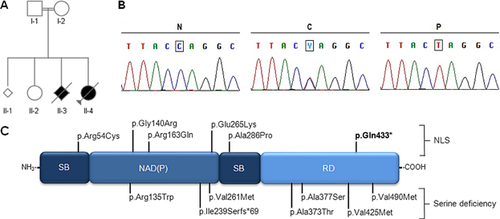
By the time this study was initiated, there were yet no genetic loci consistently associated to NLS, which led us to consider exome sequencing of the parent-offspring trio, in an attempt to identify potentially pathogenic coding variants in the fetus. However, the data subsequently published by Shaheen et al. [2014] prompted us to focus the analysis on the PHGDH gene [Shaheen et al., 2014]. Indeed, a potentially pathogenic, homozygous single-nucleotide substitution (c.1297C>T) was identified in the fetal DNA, both parents being heterozygous (Fig. 5A and B). Structurally, this mutation changes glutamine residue 433 for a premature stop codon (p.Gln433*) at the C-terminal regulatory domain of PHGDG, anticipating the deletion of the last 101 amino acids (Fig. 5C). Although no functional studies were performed, this mutation was predicted to behave as a null allele, preventing the expression of PHGDH from both alleles due to surveillance by the mRNA nonsense-mediated decay system.
DISCUSSION
Although NLS has been recognized as a distinct genetic entity for over 40 years, its molecular basis remained elusive until recently, when Shaheen et al. performed autozygosity mapping and gene sequencing in three NLS patients from distinct consanguineous Saudi Arabian families [Shaheen et al., 2014]. Prior to this study, some authors suggested that NLS might belong to the neuroectodermal dysplasia disease group [Lazjuk et al., 1979; Ejeckam et al., 1986; Naveed et al., 1990; Bronshtein et al., 1993]. Others hypothesized defects in one or more genes on the 6q and/or 9p regions, based on mouse models of restrictive dermopathies, which resemble the “cocoon”-like aspect of the fetus [Manning et al., 2004]. Hence, the association of the PHGDH locus with NLS came as a surprise [Shaheen et al., 2014], since coding variants in this gene were already known to cause PHGDH deficiency, a less severe condition characterized by delayed development, microcephaly, seizures and growth retardation [Jaeken et al., 1996; Van der Crabben et al., 2013]. Interestingly, the conceptual link between phenotype and an inborn error of metabolism in NLS was established more than two decades after its initial proposal by Shapiro et al. [1992], who argued that the abnormal fat deposits, the ichthyotic skin, and the eventual development of cataract seen in NLS should relate to an underlying metabolic defect [Shapiro et al., 1992].
Classification of NLS phenotypes was proposed by Curry [1982], who divided NLS into three subgroups according to selected clinical findings. Patients of group I would be those who present mainly with arthrogryposis, partial syndactyly, squamous skin and bone hypomineralization. Group II would include patients with severe swelling of hands and feet—also known as “glove-shaped” limbs—secondary to oedema and fat deposition, pronounced ichthyosis and poor bone mineralization. Patients with the most typical clinical findings of NLS would be placed in this group [Manning et al., 2004]. Finally, group III would be reserved for patients with hypoplastic digits, short limbs, microtia, and severe ichthyosis similar to that observed in harlequin ichthyosis. It is not known whether these phenotypic differences are due to variable expressivity of a single mutated gene, or mutations in different genes.
Besides variable expression in NLS, mutations in the phosphoserine aminotransferase (PSAT1) and phosphoserine phosphatase (PSPH) genes have also been reported, supporting the hypothesis of allelic heterogeneity for NLS. These results also show that NLS might arise from mutations in at least three different genes involved in the L-serine biosynthesis pathway, probably reflecting severe forms of milder inborn errors of metabolism [Acuna-Hidalgo et al., 2014; Shaheen et al., 2014].
In light of our results and those presented by Shaheen and colleagues and Acuna-Hidalgo and colleagues [Acuna-Hidalgo et al., 2014; Shaheen et al., 2014], we propose that at least part of the variable clinical presentations of NLS might be explained by different PHGDH mutations that give rise to a variable of disease. Accordingly, the NLS-associated variants described by Shaheen et al., who reported on patients that seem phenotypically less affected then the one we describe here, are missense substitutions not entirely different from other PHGDH missense mutations observed in individuals with the inborn error of serine metabolism known as PHGDH deficiency [Jaeken et al., 1996; Shaheen et al., 2014]. Despite missense mutations located in conserved catalytic residues of the enzyme [Shaheen et al., 2014], PHGDH might still have some degree of residual activity in those cases (although functional studies remain to be conducted to experimentally confirm this hypothesis). On the other hand, the patient reported here was shown to encode a homozygous premature termination codon mutation, which is predicted to result in complete PHGDH absence, due to the activity of the cellular nonsense mRNA-mediated decay system [Chang et al., 2007], which in turn might result in the more severe phenotype observed here.
In conclusion, we present a clinical characterization of a NLS patient using MRI evaluations. A homozygous premature stop codon mutation in the PHGDH gene was identified in the patient reported here, which corroborates a recent association of this locus with NLS [Acuna-Hidalgo et al., 2014; Shaheen et al., 2014]. The identification of additional patients will likely shed light on the phenotypic and genetic diversity of NLS, leading to a better understanding of serine-deficiency diseases and its genotype-phenotype correlations.
ACKNOWLEDGMENTS
The authors are indebted to the family reported here, for their support for publication. Part of this work was supported by the Spanish Ministry of Science and Innovation SAF2013-43365-R, the CIBERER Program of Research on Pediatric diseases (ACCI 2012), the Brazilian National Council for Research and Development (CNPq), and Hospital de Clínicas de Porto Alegre (FIPE-HCPA).