Fatigue in adults with Marfan syndrome, occurrence and associations to pain and other factors
Abstract
This study aims to investigate how fatigue affects adults with verified Marfan syndrome (MFS) in their daily lives, by examining fatigue levels and prevalence of severe fatigue compared to the general Norwegian population and individuals with other comparable chronic conditions. We investigated associations between socio-demographic characteristics, Marfan-related health problems, pain and fatigue. A cross-sectional study was conducted, using a postal questionnaire including the Fatigue Severity Scale (FSS) and questions on socio-demographic characteristics, Marfan-related health problems and pain. One hundred seventeen persons with MFS were invited to participate, 73 answered (62%). Participants reported significantly higher FSS scores and prevalence of severe fatigue compared to the general Norwegian population and patients with rheumatoid arthritis (RA), but lower than for other chronic conditions. Participants with chronic pain reported higher fatigue scores than those without chronic pain. Participants on disability benefits reported higher fatigue scores than participants who were working or enrolled in higher education. Marfan-related health problems like aortic dissection and use of blood pressure medication were not significantly associated with fatigue. In multivariable regression analyses chronic pain and employment status were significantly associated with fatigue. The final multivariable model explained 24% of the variance in fatigue scores. Our results show that fatigue is common in MFS patients and that it interferes with their daily lives. Chronic pain and employment status show significant associations to fatigue. This implies that fatigue is important to address when meeting MFS patients in clinical practice. There is need for more research on fatigue in Marfan syndrome. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Marfan syndrome (MFS) is a rare, autosomal dominant genetic connective tissue disorder caused by mutations in the fibrillin 1 gene. It is diagnosed using using diagnostic criteria; the Ghent 1 criteria from 1996 [De Paepe et al., 1996], and the Ghent 2 criteria from 2010 [Loeys et al., 2010]. MFS can affect several organ systems [De Paepe et al., 1996]. Clinical symptoms vary both between and within families, and tend to evolve with age [Loeys et al., 2010]. The most serious complication relates to the cardiovascular system with risk of dilatation and dissection of the aorta and other large vessels [Loeys et al., 2010]. Many have skeletal signs with long limbs, hypermobile joints, chest deformities, and scoliosis [De Paepe et al., 1996]. Some describe chronic pain [Peters et al., 2001a]. Visual problems are common due to lens dislocation, which entails risk for retinal detachment [De Paepe et al., 1996]. To reduce the risk of aortic dilatation and lens dislocation, many patients are advised to refrain from contact sports and to limit their physical exertion [Loeys et al., 2010]. This may lead to inactivity and a sedentary lifestyle [Rand-Hendriksen et al., 2010].
MFS patients described how fatigue limits their daily life. Few studies, however, examined the occurrence of fatigue and its possible associations in adults with verified MFS. Increased knowledge is imperative to understand fatigue in MFS patients and to design intervention strategies. Fatigue is a multifaceted concept covering physiological and psychological aspects. It is often described as an “overwhelming sense of tiredness, lack of energy and feeling of exhaustion, mental, physical or both” [Dittner et al., 2004]. Severe fatigue is common in the general population with 22% prevalence [Loge et al., 1998; Bültmann et al., 2002a; Lerdal et al., 2005]. Fatigue is associated with having a chronic health problem [Lerdal et al., 2005], occupational status [Loge et al., 1998], psychological distress [Pawlikowska et al., 1994], and low physical activity [Bültmann et al., 2002b], and is reported as a debilitating symptom in many different diseases [Dittner et al., 2004].
We identified only four studies exploring fatigue in MFS, including 260 patients total [Peters et al., 2001a; Percheron et al., 2007; Rand-Hendriksen et al., 2007; van Dijk et al., 2008]. One study found that 89% of their respondents with self-reported MFS experienced fatigue, that the experience of fatigue significantly heightened the respondent's perception of their condition's severity, and that it was significantly associated with the use of cardiovascular medication [Peters et al., 2001a]. The other three studies included participants with MFS verified by diagnostic criteria; and all report higher fatigue scores compared to healthy controls on different instruments [Percheron et al., 2007; Rand-Hendriksen et al., 2007; van Dijk et al., 2008]. Fatigue was associated with psychological distress (in women) [Rand-Hendriksen et al., 2007] and orthostatic intolerance [van Dijk et al., 2008]. The studies didn't use multivariable analyses. Methodological differences make further comparison difficult. Given the complexity of MFS, we expect that several factors, such as Marfan-related health problems, may be associated with fatigue. Our study aims to explore how fatigue affects MFS patients in their daily lives by examining the level of fatigue and prevalence of severe fatigue in adults with verified MFS.
MATERIALS AND METHODS
This cross-sectional, postal questionnaire based study is part of a larger study about challenges in education, work, and daily life for adults with MFS. The study has been approved by the Data Protection Officer at Oslo University Hospital and the Regional Ethics Committee for medical and health research ethics in eastern Norway. Permission was given to collect and analyze anonymous questionnaire data.
Participants and Procedure
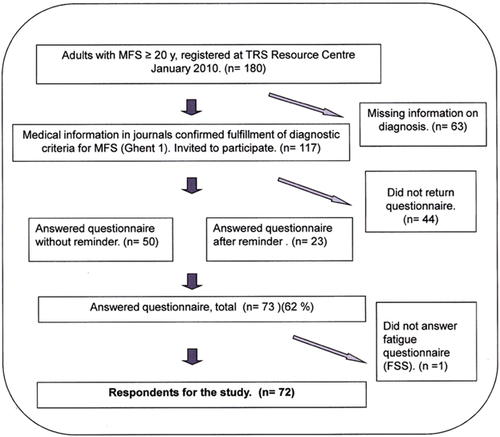
In spring 2010, we examined the patient journals of all patients age 20 and above (n = 180), registered with MFS at TRS National Resource Centre for rare disorders in Norway. All patients with documentation confirming that they fulfilled the Ghent 1 criteria (the available criteria at the time) (n = 117) were invited to participate in the study and received a consent form and questionnaire by post. After 6 weeks patients who had not responded received a reminder with a new copy of the questionnaire. Seventy-three persons returned the questionnaires (response rate of 62%). One respondent's questionnaire missed all items on the FSS, and was omitted from this part of the study. Figure 1 shows a flow chart of the recruitment of participants.
Assessment Methods
Fatigue was assessed with the Fatigue Severity Scale (FSS). This is a nine-item questionnaire developed to measure the impact of fatigue on daily functioning [Krupp et al., 1989]. The FSS is widely used and has been found valid and reliable in different patient groups [Whitehead, 2009]. Each item is scored on a seven-point Likert scale with a range from 1 (completely disagree) to 7 (completely agree). To assess how fatigue affects MFS patients in their daily lives, we analyzed the distribution of scores for single items.
To assess level of fatigue we calculated a FSS mean score of all nine items for each respondent, ranging from 1.0 (i.e., no fatigue) to 7.0 (i.e., maximum fatigue). Minimal clinically important difference (MCID) for FSS mean scores have not been published for MFS patients, but are reported to be 0.4 points for patients with systemic lupus erythematosus (SLE) [Goligher et al., 2008] and 0.7 points for patients with RA [Pouchot et al., 2008].
To assess the prevalence of “severe fatigue” versus “no fatigue,” the following cut-off values were used: Non-fatigue = FSS mean score ≤4; severe fatigue = FSS mean score ≥ 5; and borderline fatigue = FSS mean score >4 and <5 [Roelcke et al., 1997; Bakshi et al., 1999; Lerdal et al., 2005].
To evaluate the impact of fatigue, we compared FSS mean scores for our participants with what previous studies using FSS, have reported for: (1) the general Norwegian population [Lerdal et al., 2005], (2) Marfan patients [Percheron et al., 2007; Rand-Hendriksen et al., 2007], and (3) studies of patient groups with comparable chronic diseases. The latter are studies on patients with musculoskeletal pain; RA [Mancuso et al., 2006], and Ehlers–Danlos syndrome hypermobility type/joint hypermobility syndrome (EDS-HT/JHS) [Celletti et al., 2013] and patients with a rare and heritable disease called late onset Pompe disease (PD), a slowly progressive proximal myopathy that affects mobility and respiration [Hagemans et al., 2007].
The questionnaire included questions addressing demographics (i.e., gender, age, age at MFS diagnosis, education level, employment status, living alone, or with partner) as well as Marfan-related health problems (i.e., aortic dilatation, aortic dissection, prior aortic surgery, use of blood pressure medication, and visual impairment due to lens dislocation or retinal detachment). The main study included the Standardized Nordic questionnaire [Kuorinka et al., 1987; Svebak et al., 2006], which measures the presence, location, and impact of musculoskeletal pain. To measure chronic pain we used responses to the question, “Have you during the last year continuously for at least 3 months suffered from pain or stiffness in muscles and joints?” from this questionnaire.
Statistics
Descriptive data are given as frequencies, percentages, standard deviations (SD), and mean differences with 95% confidence intervals (CI) or medians and ranges. Student's t-tests were used to compare the sample's mean fatigue score to those reported for the general population, other Marfan samples and other patient groups. Multiple linear regression analysis was used to study the association between fatigue, socio-demographic variables, Marfan-related health problems and chronic pain. Associations between independent variables and the dependent variable (mean fatigue) were examined with Pearson's correlation coefficients and Independent samples t-tests. Independent variables showing a significant relationship with the dependent variable were entered into a multiple regression model. The best subsets of independent variables were selected by excluding variables with the least contribution to the model (i.e., with the largest P-values). Residuals were examined to check mode assumptions. The statistical analyses were conducted in SPSS version 19 and a significance level of P < 0.05 was used. One respondent had two FSS items missing, while another three had one item missing. Missing items were replaced by the case substitution method that replaces missing values with the mean of the respondent's completed items [Polit and Beck, 2008; Lerdal et al., 2010].
RESULTS
Of the 72 respondents, 41 were women (57%). Table I displays respondents socio-demographic characteristics, prevalence of Marfan-related health problems and chronic pain.
| Mean (SD) | Median (range) | |
|---|---|---|
| Socio-demographic features | ||
| Age in years | 44.2 (13.1) | (20–71) |
| Age in years at diagnosis (n = 67)a | 24.0 | (0c–56) |
| n (%) | ||
| Women | 41 (57) | |
| Living with adult partner | 42 (58) | |
| Education level (highest finished education): ≤13 years | 39 (54) | |
| Employment status (employed or under higher education)b | 41 (57) | |
| Marfan-related health problems | ||
| Dilated aorta | 65 (90) | |
| Aortic dissection | 25 (35) | |
| Operation aorta/other blood vessels | 42 (58) | |
| Use of blood pressure medication | 49 (68) | |
| Visual impairment due to lens dislocation or retinal detachment | 25 (35) | |
| Chronic pain/stiffness in muscles and joints | 46 (64) | |
- a Five persons did not know their age at diagnosis.
- b Employed or under higher education versus on disability benefits.
- c Diagnosis at birth because of clinical signs and diagnosed parent.
Non-respondents (n = 44) were not significantly different from respondents regarding gender (29 women, 67%), or age (mean = 41.4, range 20–76). The FSS item with highest mean score was, “My motivation is lower when I am fatigued,” for which 86% of respondents scored ≥5. Sixty percent of respondents scored five or higher on the item, “Fatigue interferes with my work, my family, or social life” (Table II).
| Item | Mean | Score distribution for scores 1–7 in % | ||
|---|---|---|---|---|
| 1–3 | 4 | 5–7 | ||
| My motivation is lower when I am fatigued | 5.8 | 8 | 6 | 86 |
| Fatigue interferes with my physical functioning | 5.4 | 12 | 10 | 78 |
| Fatigue interferes with my work, family or social life | 4.8 | 28 | 12 | 60 |
| Fatigue is among my most debilitating symptoms | 4.8 | 35 | 8 | 57 |
| Fatigue interferes with carrying out certain duties and responsibilities | 4.7 | 30 | 10 | 60 |
| I am easily fatigued | 4.7 | 25 | 15 | 60 |
| My fatigue prevents sustained physical functioning | 4.4 | 38 | 11 | 51 |
| Exercise brings on my fatigue | 3.9 | 42 | 19 | 39 |
| Fatigue causes frequent problems for me | 3.9 | 46 | 18 | 36 |
- Item scores range from 1 (strongly disagree) to 7 (strongly agree). The items are ranked by descending mean scores.
A comparison of mean FSS scores for the study sample to reported values from previously published studies of the general Norwegian population, other MFS studies, and other patient groups is shown in Figure 2. The mean FSS score for the study group (4.72, SD 1.40) is significantly higher than for the general Norwegian population [Lerdal et al., 2005] and for patients with RA [Mancuso et al., 2006], not significantly different from the MFS study by Rand-Hendriksen et al. [2007], but significantly lower than for the other MFS group [Percheron et al., 2007], for patients with EDS-HT/JHS [Celletti et al., 2013] and patients with PD [Hagemans et al., 2007] (Table III).
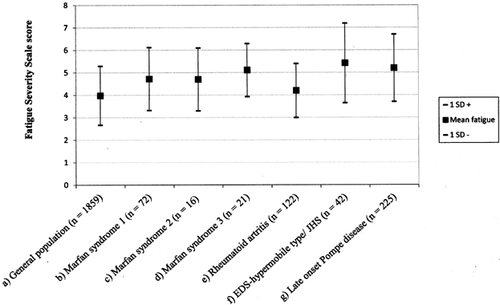
| Mean FSS score | SD | Mean difference | CI, mean difference | P-value (two-tailed) | |
|---|---|---|---|---|---|
| Current study | 4.72 | 1.40 | |||
| General population Lerdal et al. [2005] | 3.98 | 1.31 | 0.74 | 0.47, 1.07 | ≤0.001* |
| Rheumatoid Artritis Mancuso et al. [2006] | 4.20 | 1.20 | 0.52 | 0.19, 0.85 | 0.003* |
| Marfan syndrome Rand-Hendriksen et al. [2007] | 4.70 | 1.4 | 0.21 | −0.31, 0.35 | 0.90 |
| Marfan syndrome Percheron et al. [2007] | 5.11 | 1.18 | −0.39 | −0.72, −0.06 | 0.02* |
| Pompe disease Hagemans et al. [2005] | 5.20 | 1.50 | −0.48 | −0.81, −0.15 | 0.005* |
| EDS-HT/JHS Celletti et al. [2013] | 5.40 | 1.77 | −0.68 | −7.01, −0.35 | ≤0.001* |
- * Significant.
Forty-two percent of respondents had a mean FSS score of five or higher (indicating severe fatigue), while 29% had a score between four and five (indicating borderline fatigue) and 29% had a score equal to or under four (indicating non-fatigue). In initial bivariate analyses, FSS scores had a significant but weak positive correlation to age (r = 0.25, P = 0.03) but not to age when diagnosed with MFS (r = 0.11, P = 0.35). FSS scores were significantly associated with gender, employment status, and chronic pain but not to other variables (Table IV).
| Independent variables | Mean FSS score (SD) | Mean difference (95% CI) | Independent samples t-test | P-value (two-tailed) |
|---|---|---|---|---|
| Gender | ||||
| Women | 5.04 (1.40) | 0.73 (−1.39, −0.09) | t = 2.30 | 0.03* |
| Men | 4.30 (1.32) | |||
| Living alone/with partner | ||||
| Living alone | 4.74 (1.40) | 0.07 (−0.61, 0.76) | t = 0.21 | 0.96 |
| Living with adult partner | 4.70 (1.44) | |||
| Education level (highest finished education) | ||||
| Education ≤13 years | 4.97 (1.35) | 0.55 (−0.09, 1.21) | t = 1.70 | 0.09 |
| Higher education/university | 4.42 (1.43) | |||
| Employment status | ||||
| On disablement benefits | 5.40 (1.30) | 1.40 (0.52, 1.75) | t = 3.70 | ≤0.001* |
| Employed/under higher education | 4.20 (1.31) | |||
| Dilated aorta | ||||
| No | 5.46 (1.35) | 0.85 (−0.33, 2.02) | t = 1.43 | 0.16 |
| Yes | 4.62 (1.38) | |||
| Aortic dissection | ||||
| No | 4.46 (1.40) | −0.65 (−1.32, 0.03) | t = −1.90 | 0.06 |
| Yes | 5.10 (1.29) | |||
| Operation on aorta or other blood vessels | ||||
| No | 4.79 (1.34) | 0.17 (−0.51, 0.84) | t = 0.50 | 0.62 |
| Yes | 4.62 (1.43) | |||
| Use of blood pressure medication | ||||
| No | 4.70 (1.45) | −0.03 (−0.74, 0.68) | t = −0.08 | 0.94 |
| Yes | 4.72 (1.40) | |||
| Visual impairment | ||||
| No | 4.59 (1.35) | −0.37 (−1.08, 0.33) | t = −1.06 | 0.29 |
| Yes | 4.97 (1.54) | |||
| Chronic pain | ||||
| No | 4.00 (1.38) | 1.12 (−1.76, −0.48) | t = −3.50 | ≤0.001* |
| Yes | 5.12 (1.26) | |||
- * Significant.
In multivariable regression analyses we first included age, gender, education level, chronic pain, and employment status. After controlling for age and gender, only chronic pain and employment status remained significant. Persons with chronic pain had higher levels of fatigue than persons without chronic pain. Participants with disability benefits had higher fatigue scores than those working or under higher education. The final multivariable regression model (Table V) explained 24% of the variance in FSS scores (adjusted R2 = 0.24).
| Crude estimates | Adjusted estimatesa | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | |
| Age | 0.03 | 0.00, 0.05 | 0.03* | — | — | — |
| Gender | 0.73 | 1.39, 0.09 | 0.03* | — | — | — |
| Education level | −0.56 | −1.22, 0.10 | 0.09 | — | — | — |
| Employment status | −1.14 | −1.75, −0.52 | ≤0.001* | −0.88 | −1.54, −0.22 | 0.009* |
| Aortic dissection | 0.65 | −0.03, 1.32 | 0.06 | — | — | — |
| Chronic pain | 1.12 | 0.50, 1.80 | 0.001* | 0.88 | 0.27, 1.50 | 0.005* |
- Age in years; gender: women = 0, men = 1; education level (highest finished education): 0 ≤ 13 years, 1 > 13 years; employment status: 0 = disability benefits, 1 = working or under higher education; aortic dissection: 0 = no, 1 = yes; chronic pain: 0 = no, 1 = yes.
- a Estimated regression coefficients β with 95% confidence intervals and P-values for the final multivariate regression model.
- * Significant.
DISCUSSION
This study found that many adult MFS patients experience fatigue interfering with daily activities. Levels of fatigue and prevalence of severe fatigue are higher in these adult MFS patients compared to the general Norwegian population [Lerdal et al., 2005], suggesting that fatigue significantly impacts their daily live.
Single-item distribution analysis revealed that 60% and 57%, respectively, had a mean score ≥5 for the items, “fatigue interferes with my work, family, and social life” and “fatigue is among my most debilitating symptoms.” This underscores that fatigue interferes with daily life for adults with MFS, and that it is important to address fatigue in clinical settings. Interestingly, the question “exercise brings on my fatigue” is the item with lowest mean score, and only 39% had a mean FSS score ≥5. In comparison Hagemans et al. [2007] found that 84% of patients with late onset PD had a mean FSS score ≥5 on this item. Late onset PD entails weakness of the skeletal and respiratory muscles and possibly more severe physical disability than MFS. The disparity in findings may therefore be explained by differences in physical disability. However, one might ask if this is related to the advice on physical restrictions given to many MFS patients to prevent medical complications. One hypothesis is that many MFS patients perform only low intensity exercises, as reported by Peters et al., [2001b], and thus do not experience that exercise brings on fatigue. It is important to note that the item “fatigue interferes with my physical functioning” has the second highest mean in the MFS group and 78% have a mean FSS score ≥5. This shows that physical functioning may be troublesome and connected to fatigue.
Our participants report significantly higher fatigue compared to the general Norwegian population [Lerdal et al., 2005] and patients with RA [Mancuso et al., 2006]. These differences also exceed MCID values proposed for other patient groups [Goligher et al., 2008; Pouchot et al., 2008], indicating that the differences are clinically relevant. However, our participants have lower mean FSS score than patients with PD [Hagemans et al., 2007] and EDS-HT/JHS [Celletti et al., 2013]. The latter, also a connective tissue disorder, is associated with hypermobility and musculoskeletal pain [Rombaut et al., 2010], but not with life-threatening complications. Therefore it is somewhat surprising that patients with EDS-HT/JHS have significantly higher fatigue scores than MFS patients. This might indicate that factors other than disease severity contribute to fatigue.
Forty-two percent of the participants reported severe fatigue [FSS mean score ≥ 5], which is higher than in the general Norwegian population (23%) [Lerdal et al., 2005] and for patients with RA (30%) [Mancuso et al., 2006]. By contrast, 67% of patients with late onset PD reported severe fatigue [Hagemans et al., 2007]. However, the variance in fatigue scores in adults with MFS are large, and approximately one third (29%) of participants reported non-fatigue (FSS mean score ≤ 4) compared to 53% in the general Norwegian population [Lerdal et al., 2005].
Interestingly, we found no significant associations between fatigue scores and Marfan-related health problems (i.e., aortic dilatation, aortic dissection, aortic surgery, and visual impairment due to lens dislocation or retinal detachment). We expected these factors to be of importance. Since the group without aortic dilation was small, this result should be interpreted with caution. We found no significant associations between fatigue and the use of blood pressure medication. The mean FSS score was not significantly different in the group using blood pressure medication (4.72), compared to the group who did not (4.70) (Table I). This contrasts the results from Peters et al. [2001a, 2001b], who found significant associations between cardiovascular medication use and fatigue. The discrepancy might derive from methodological factors; such as inclusion criteria, disease characteristics, and use of different questionnaires. Since questions about type and dosage of blood pressure medication were not included in our study, differences in fatigue levels related to types and dosage of the medications cannot be analzyed. Further comparison with results from the study by Peters et al. [2001a, 2001b] is also difficult.
It has been an accepted fact that there is a correlation between use of beta-blockers and fatigue [Ko et al., 2004]. Two systematic review studies dispute this view [Ko et al., 2002, 2004]. Meta-analyses showed no absolute risk of fatigue associated with beta-blocker therapy in patients with heart failure [Ko et al., 2004], and only a small increase in patients with myocardial infarction [Ko et al., 2002]. This underscores the need for studies investigating the association between fatigue and use of blood pressure medications in MFS.
In our multivariable analyses, chronic pain and employment status show significant associations to fatigue. Pain is most strongly associated to fatigue. Sixty-four percent of participants reported chronic pain. This is higher than for the general Norwegian population with 45% reporting chronic pain/stiffness in muscles and joints [Svebak et al., 2006]. In comparison, Peters et al. [2001a] found that 90% of their respondents with MFS experienced pain on a regular basis. This illustrates that the prevalence of pain may vary in different Marfan populations. Pain is also highly prevalent in EDS-HT [Voermans et al., 2010; Rombaut et al., 2011]. EDS-HT shares some signs with MFS, like hypermobility. To our knowledge the association between hypermobility, fatigue, and pain in connective tissue disorders has not been extensively studied.
We didn't find articles exploring associations between fatigue and pain in MFS patients. In other patient groups, such as RA, studies report conflicting results. Mancuso et al. [2006] found significant associations in employed RA patients between FSS scores and pain in bivariate, but not in multivariable analyses. In contrast, Pollard et al. [2006] found pain to be the factor most strongly associated with fatigue in multivariable analyses in a study of RA patients ages 22–91 years. The disparity between the studies may be caused by methodological differences. In patients with ankylosing spondylitis pain was the factor most strongly associated to fatigue in multivariable analyses, and the authors suggest that treatment of fatigue should focus on pain management [Brophy et al., 2013]. In our study, receiving disability benefits was associated with higher mean FSS scores than being employed or enrolled in higher education. This result concurs with that for the general Norwegian population [Loge et al., 1998], in which people receiving disability benefits and being unemployed reported the highest levels of fatigue. Our study was cross-sectional, hence we could not explore causal associations. Future studies should investigate whether either work or disease-specific variables explain why persons with MFS are unable to work and if this is associated to fatigue.
The final multivariable model explains 24% of the variance in fatigue scores. This implies that other factors contribute to fatigue. Given the complexity of fatigue and MFS, this is not surprising. One factor of interest is depression, since association between depression and fatigue has been reported [Ferentinos et al., 2011]. MFS is a disease with possible life-threatening complications, and many may experience psychological challenges. Rand-Hendriksen et al. [2007] found that psychological distress can be associated with fatigue in women with MFS, but argue that their study was too small to conclude with certainty. Peters et al., [2001a] did not find associations between fatigue and depression in MFS patients Thus, current knowledge on fatigue and psychological issues in MFS is diverging.
Other factors possibly associated to fatigue in MFS are sleep disturbance and physical activity. Sleep disturbances are common in patients with chronic pain conditions [Menefee et al., 2000] and associated with fatigue in patients with multiple sclerosis [Kaminska et al., 2012]. Treatment of obstructive sleep apnea has been found effective for treating fatigue [Chotinaiwattarakul et al., 2009]. Sleep disturbances like obstructive sleep apnea are common in MFS patients [Rybczynski et al., 2010], and the possible association between fatigue and sleep apnea should be investigated.
Being physically inactive during leisure time is an important predictive factor for fatigue in healthy male employees [Bültmann et al., 2002b]. The association between physical activity and fatigue has not been explored in MFS patients. This might be important, since MFS patients often are advised to limit their physical exertion. In other patient groups physical activity and training has been found effective for treating fatigue [Neill et al., 2006; Dalgas et al., 2010].
Implications for Further Research
This study has shown that fatigue is prevalent in MFS, and that more exploration is needed. We suggest a study combining clinical examination of MFS patients according to the Ghent 2 features with a questionnaire study including questions regarding fatigue, type and dosage of blood pressure medication, demographic features, musculoskeletal pain, sleep disturbances, physical activity, depression, and psychological distress.
Implications for Clinical Practice
Our results show that fatigue is common and interferes with daily life for patients with MFS. Asking about fatigue and possible associated factors like chronic pain and employment status should therefore be part of the routine when examining MFS patients. However, the multivariable model explained 24% of the variation in fatigue scores, implying that other factors contribute to fatigue in MFS. A thorough examination of all possible aspects that might influence fatigue is therefore important. The cross sectional design of this study does not give information on causes to fatigue, and thus limits evidence on treatment options. Possible interventions discussed for other patient groups include energy conserving strategies [Mathiowetz et al., 2005], physical activity [Garssen et al., 2004] and treating sleep apnea [Chotinaiwattarakul et al., 2009].
Strengths and Limitations
The study benefits from including patients with MFS verified by diagnostic criteria. The cohort is relatively large compared to other studies of fatigue in MFS. Sixty-two percent of adults registered with verified MFS, age ≥20 years, at TRS Resource Centre participated. One limitation is that the total number of MFS patients is unknown; hence it is uncertain if the respondents were representative for the MFS population in Norway. Another possible limitation is that we used self-constructed questions addressing Marfan-related health problems. This was necessary since no validated instruments exist. The use of multivariable analysis of fatigue and associated factors strengthens the study, as this has not been done previously.
CONCLUSION
This study confirms that fatigue affects persons with MFS by interfering with their daily lives. Their level of fatigue and the prevalence of severe fatigue are higher than reported for both the general population and a study sample of RA patients, but lower than reported for groups of patients with other chronic conditions. Multivariable regression analyses reveal that chronic pain and employment status show significant associations to fatigue. There is need for more research on fatigue in Marfan syndrome.
ACKNOWLEDGMENTS
The authors want to thank the Norwegian Marfan Association and all participants who made this study possible.