Stillbirth: The heart of the matter
Abstract
We evaluated 2,083 cases within the Wisconsin Stillbirth Service Program (WiSSP) that had autopsy reports or ultrasound data relevant to the heart. Of these, 167/1,782 (9.4%) stillbirths after 20 weeks and 11/301 (3.7%) miscarriages <20 weeks had congenital heart disease (CHD). Cases were classified by type of heart defect and whether it related to cause of death. Among cardiac anomalies that contributed significantly to fetal death, 125/151 (83%) were associated with underlying conditions or syndromes, nearly half of which were chromosomal. The most common forms of CHD in stillborns were severe cyanotic lesions (3%), then ventricular (2.6%) and atrial (1.9%) septal defects. Compared to livebirths, this represents a shift toward more severe cardiac lesions, although all comparable categories, including non-lethal conditions such as atrial septal defect, are more common in stillbirths. Clinical cardiomyopathy was identified as cause of death in 1.2% of stillborns. Cardiomegaly, occurring in 26.7% of all cases and 76.7% of infants born to diabetic mothers, may represent undiagnosed cardiomyopathy and/or may decrease fetal tolerance of hypoxia. In contrast, 78.5% of Turner syndrome infants, all <32 weeks, had small hearts. More attention to cardiac findings can lead to increased understanding of stillbirth causes. Based on our findings, we recommend chromosome studies on all stillbirths and close attention to the heart during second trimester ultrasounds, with chromosome studies offered if CHD is found. Consideration of heart size can result in prenatal identification of infants at risk for stillbirth, particularly large hearts in fetuses of diabetic mothers in the third trimester, which may identify fetal cardiomyopathy before it becomes life-threatening. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Stillbirth, defined as fetal death that occurs at 20 weeks of gestational age or later, is a fairly common event, accounting for about 26,000 fetal deaths in the United States each year, and occurring in about 1 in 160 pregnancies [Macdorman and Kirmeyer, 2009]. Second trimester miscarriages are even more frequent, occurring in 3% of pregnancies [Dukhovny et al., 2009]. Congenital heart disease (CHD) is the most prevalent congenital disorder in liveborn infants and is the most frequent cause of infant death resulting from birth defects [Yang et al., 2006; Tennant et al., 2010; Roger et al., 2012], yet very little research has specifically addressed cardiac malformations in stillborn fetuses. Furthermore, to our knowledge, there has been no detailed analysis of the causal or contributory role of CHD to fetal death.
The generally accepted estimate of CHD prevalence in livebirths is 0.8%, with a range of 0.4–5.0% depending on the lesions included and the method of ascertainment [Hoffman and Kaplan, 2002; Bernier et al., 2010]. Using echocardiography to screen asymptomatic newborns, Ishikawa et al. [2011 identified cardiac defects in 5%, which was much higher than other studies, presumably due to the inclusion of infants who were asymptomatic and did not require invasive treatment. The frequency of congenital heart disease in autopsied stillbirths over 20 weeks ranges from 2.1% [Samánek et al., 1985] to 7.7% [Hoffman and Christianson, 1978] (Table I). In miscarriages prior to 20 weeks, the range is even greater, from 2% [Ursell et al., 1985] to 18% [Gerlis, 1985] though the latter study is not strictly comparable due to the use of serial sectioning and microscopic evaluation of the smallest hearts. Although ascertainment of cardiac anomalies may be different at autopsy compared to clinical studies in living infants, it is clear that congenital heart disease is more prevalent in stillbirths and miscarriages than in livebirths. Furthermore, the increase is greatest in more severe cardiac lesions [Hoffman and Christianson, 1978].
| Study | Gestational age | Method | Cardiac defects |
|---|---|---|---|
| Samánek et al. [1985 | >28 weeks | Autopsy | 81/3,962 = 2.1% |
| Mitchell et al. [1971 | >20 weeks | Autopsy | 37/1,344 = 2.75% |
| Bound and Logan [1977 | “Stillbirth” | Autopsy | 87/997 = 5.7% |
| Hoffman and Christianson [1978 | >20 weeks | Autopsy | 17/221 = 7.7% |
| Ursell et al. [1985 | 20–28 weeks | Autopsy | 3/75 = 4% |
| Ursell et al. [1985 | <20 weeks | Autopsy | 7/337 = 2% |
| Gerlis [1985 | >20 weeks | Autopsy | 3/42 = 7% |
| Gerlis [1985 | <20 weeks | Serial section | 32/179 = 18% |
| Chinn et al. [1989 | 9–40 | Autopsy | 52/400 = 13% |
Fetal cardiomyopathy can also contribute to perinatal morbidity and mortality. Cardiomyopathy is rare in liveborn infants, ranging from 4.1/100,000 livebirths [Arola et al., 1997] to around 8/100,000 livebirths [Lipshultz et al., 2003; Nugent et al., 2003]. Hypertrophic cardiomyopathy is common in infants of diabetic mothers (IDMs), being identifiable on neonatal echocardiogram in 38% of an unselected series of 100 IDMs [Abu-Sulaiman and Subaih, 2004]. Vielle et al. [1992 found ventricular septal hypertrophy on fetal echocardiography in 75% of pregnancies in diabetic women. Autopsy data differ from fetal echocardiography in that heart weight is usually measured and compared to that of other organs, but ventricular and septal thickness are not strictly comparable to the dynamic measurements at echocardiography. In a study of stillborn infants without other birth defects, Russell et al. [2008 compared heart size in IDMs with large for gestational age (LGA) and appropriate gestational age (AGA) stillbirths to non-diabetic mothers. Cardiac weight was significantly greater in the IDMs than in the LGA non-diabetic group and was entirely normal in the AGA non-diabetic group. Cardiomyopathy and/or cardiomegaly is common in IDMs and may be a contributing factor to the known increase in stillbirth with maternal diabetes [Dudley, 2007].
On the other end of the spectrum, although there is evidence that alterations in genes responsible for cardiac development can result in lethal cardiac hypoplasia in experimental animals [Cai et al., 2005]; studies of cardiac hypoplasia in humans have been very limited. Barr and Oman-Ganes [2002 suggested that even in comparison with other fetuses that have hydrops or cystic hygroma, fetuses with Turner syndrome are more likely to have cardiac hypoplasia. More recently Riggs et al. [2011 discussed asymmetric cardiac hypoplasia in fetuses with right or left outflow tract obstruction.
The aims of the present study were to provide an analysis of congenital heart malformations in stillborn fetuses and their potential to cause or contribute to fetal death, to determine the incidence of cardiomyopathy/cardiomegaly as well as cardiac hypoplasia in stillborn infants, and to investigate the role that abnormalities of cardiac size may play in contributing to fetal death. The ultimate goal was to use this knowledge to increase understanding of the causes of stillbirth and to possibly alter pregnancy management by giving more attention to cardiac findings.
METHODS
The Wisconsin Stillbirth Service Project (WiSSP) was initiated in 1983 and has reviewed over 2,600 fetal deaths. Most of these deaths (85%) are stillbirths (≥20 weeks gestational age), although second trimester miscarriages (13–19 weeks gestational age), immediate neonatal death (death <24 hr after birth), and certain cases of termination in which the fetus had complications incompatible with life (anencephaly, bilateral renal agenesis, trisomy 13 and 18) were also included. Cases are referred from more than 100 Wisconsin birthing centers and from out-of-state upon request. Although less than half of all stillbirths in the state are evaluated through WiSSP, comparison of WiSSP referrals with fetal death reports, as well as comparison of the rates of fetal anomalies identified between hospitals that refer the majority of their stillbirths with those that only occasionally refer [Pauli et al., 1994], shows no evidence of bias toward referral of stillbirths with known malformations. The neonatal deaths and terminations admittedly are referred primarily due to obvious malformations; however, these groups combined accounted for only 8% of the total WiSSP referrals, and the frequency of cardiac anomalies did not differ from that for the stillbirths, so they were included in the analysis. Second trimester miscarriages (less than 20 weeks) are included in WiSSP, because the causes and epidemiology are more similar to stillbirth than to first trimester losses [VanderWielen et al., 2011] but clearly have a lower incidence of cardiac anomalies, suggesting incomplete ascertainment of cardiac malformations in this group and thus they are separated for most of the analyses.
In accordance with the WiSSP protocol, studies are carried out at the birthing centers and all relevant information is sent for review by a WiSSP geneticist (Richard Pauli, M.D. 1983 to April 2009; Elizabeth McPherson, M.D. April 2009 to present). This information includes perinatal and family history, clinical examination, autopsy, placental examination, photographs, radiology, laboratory tests (Kleihauer Betke, etc.), and chromosome studies. Since the primary purpose of WiSSP is service-oriented, files with incomplete information are also accepted. Autopsy was completed in about 80% of cases. Chromosome studies were attempted in 90%, but due to culture failures in the earlier years before fluorescence in situ hybridization became available, results were available for only about 66%. All reviewed data are entered into the WiSSP database using FileMaker Pro software, where it is used for further studies. After reviewing all available information, the WiSSP geneticist compiles a report suggesting further studies/tests and giving a stillbirth recurrence risk that is sent to the referring physician and family upon request. Genetic counseling is available to families if desired.
This study investigated 2,082 cases that had autopsy or ultrasound data regarding the fetal heart. Autopsies were carried out by pathologists at the birthing hospitals or, if unavailable, at a central location. Cases were reviewed by a medical student (Jorgensen) under the supervision of a clinical geneticist (McPherson). Causes of death and non-causal abnormalities were attributed to the fetus, placenta, and/or mother in the same fashion as previously reported by VanderWielen et al. [2011. Briefly, anomalies were considered causal only if they could independently account for the death of the fetus. In cases where causes in two categories (e.g., fetal and placental, fetal and maternal, etc.) could each independently explain death, both were counted. Cases that had no identifiable cause of fetal death were categorized as unknown. Non-causal abnormalities that potentially contributed to fetal death were also attributed to the fetus, placenta, and mother. Fetuses whose weights were large (>97th centile) or small (<3rd centile) for gestational age were considered abnormal but not causal, as were large/small placentas (>95th centile or <5th centile) and fetal hearts that had absolute cardiomegaly (heart weight >95 centile) or relative cardiomegaly (gestational weight of the heart differs by more than 2 weeks from the gestational weight of the baby). Body weights ≥25 weeks gestational age were compared to intrauterine growth charts of Usher and McLean [1969, while body weights <25 weeks gestational age and all heart weights were compared to intrauterine growth charts of Maroun and Graem [2005.
We adhere to the definition of CHD proposed by Mitchell et al. [1971, that is, “a gross structural abnormality of the heart or intrathoracic great vessels that is actually or possibly of functional significance.” This definition excludes abnormalities of the great veins (e.g., persistent left superior vena cava) and systemic artery branches (e.g., combined brachiocephalic-left carotid arterial trunk). This definitions also excludes connective tissue anomalies such as dilated aortic root or mitral valve prolapse and bicuspid aortic valve. Multiple cardiac anomalies in the same child, unless part of a recognized constellation such as hypoplastic left heart or tetralogy of Fallot, were tabulated separately. Thus the totals for specific anomalies are not mutually exclusive. Cardiomyopathy and arrhythmias are not considered CHD, but are reported separately.
RESULTS
We evaluated 2,083 cases (1,782 stillbirths and 301 second trimester miscarriages) that had autopsy reports or relevant ultrasound data. Of these, 179 (8.5%) had CHD including 168/1,782 stillbirths (9.4%) and 11/301 miscarriages (3.7%). Gestational ages were divided into groups of 4 weeks (Table II). The incidence of CHD increased from 2.3% (13–16 weeks) to a maximum of 11% at 29–32 weeks, and then slowly declined to 9% at term.
| GA range | % Stillbirths with CHD |
|---|---|
| 13–16 | 2.3 |
| 17–20 | 4.7 |
| 21–24 | 6.9 |
| 25–28 | 10.7 |
| 29–32 | 11.2 |
| 33–36 | 10.6 |
| 37–40 | 9.6 |
| >40 | 8.6 |
The most common types of CHD were severe cyanotic lesions combined (including transposition, truncus arteriosus, hypoplastic left heart, hypoplastic right heart, Ebstein anomaly, single ventricle, double outlet right ventricle, Tetralogy of Fallot, and severe pulmonic stenosis), VSD, and atrial septal defects (ASD), with overall incidences of 3.0%, 2.6%, and 1.9%, respectively. The incidence of specific lesions compared to rates in the liveborn population [Bjornard et al., 2013] is shown in Table III. All cardiac malformations are more common in our stillbirth cohort with stillbirth:livebirth ratios ranging from 58 for Ebstein's anomaly to 4.75 for coarctation.
| Heart defect | Total | % of all SB (n = 2,082) | Upper quartile of LB (%) Hoffman and Kaplan [2002 | SB:LB ratio |
|---|---|---|---|---|
| Cyanotic (combined) | 61 | 2.95 | 0.1533 | 19.1 |
| Truncus arteriosus | 7 | 0.34 | 0.0136 | 24.7 |
| Ebstein anomaly | 6 | 0.29 | 0.0161 | 17.9 |
| Single ventricle | 5 | 0.24 | 0.0136 | 17.7 |
| Hypoplastic left heart | 3 | 0.15 | 0.0279 | 15.5 |
| Hypoplastic right heart | 7 | 0.34 | 0.0224 | 15.0 |
| Transposition | 10 | 0.48 | 0.0388 | 12.4 |
| Tetralogy of Fallot | 9 | 0.44 | 0.0577 | 7.5 |
| DORV | 2 | 0.10 | 0.0245 | 3.9 |
| Pulmonic stenosis | 6 | 0.29 | 0.0836 | 3.4 |
| Ventricular septal defect | 54 | 2.61 | 0.4482 | 5.8 |
| Atrial septal defect | 37 | 1.78 | 0.1059 | 16.8 |
| Atrioventricular canal | 10 | 0.48 | 0.038 | 12.6 |
| Coarctation | 4 | 0.19 | 0.040 | 4.75 |
| Aortic stenosis | 2 | 0.10 | 0.011 | 9.09 |
- CDC, center of disease control, SB, stillbirth; LB, live births; DORV, double outlet right ventricle.
Of the 179 babies with CHD, 157 (88%) had fetal causes of death, 22 (12%) had placental causes of death, 6 (3%) had maternal causes of death, and 16 (9%) had unknown causes of death (categories not mutually exclusive). This stands in contrast to the entire group of stillborns with cardiac data in which the causes of death are fetal (26.8%), placental (25.5%), maternal (10.2%), and unknown (42.6%). Among the 179 stillborn infants with CHD, the CHD was causal in 151 (84%), of which 125 (83%)were associated with other anomalies, and 26 (17%) were isolated. The remaining 28 babies (17% of the total) had incidental CHD; 23 (82%) of these were isolated, and in the remainder, the CHD was unrelated to a syndrome identified in the infant (Fig. 1). In the group with cardiac anomalies, the most prevalent causes of fetal death included chromosomal syndromes (58/151 = 38%), other known syndromes (43/151 = 28.5%), non-syndromic isolated heart defects (27/151 = 18%), multiple anomalies not fitting any known syndrome 12/151 = 8%), and ectopia cordis (10/151 = 7%). Placental causes of death included placental insufficiency, twin–twin transfusion, and abruption. All maternal causes of death were from premature rupture of membranes/chorioamnionitis.
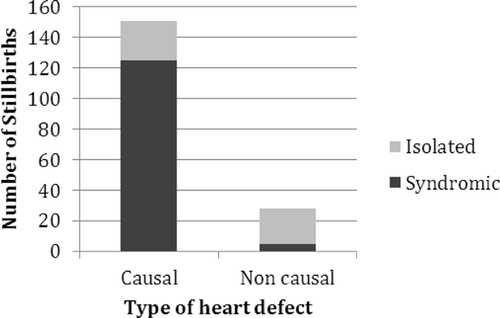
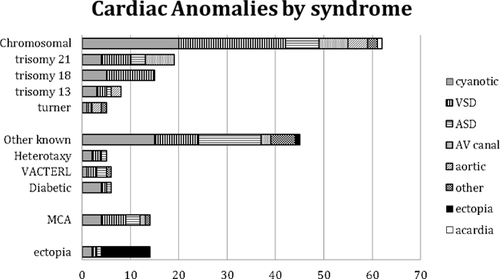
Congenital heart defects with no associated extracardiac malformations were considered isolated even if the infant was small for gestational age. Associated anomalies were divided into six groups, including chromosomal (59/130 = 45%), other known syndromes (43/130 = 33%), multiple congenital anomalies not fitting a known syndrome (12/130 = 9%), ectopia cordis (10/130 = 8%), acardiac twin (4/130 = 3%), and tumor 3/130 = 2%). The diseases and conditions associated with each category are shown in Figure 2. The most common chromosomal disorders were trisomy 21 and trisomy 18, while the most common specific non-chromosomal syndromes were VACTERL (vertebral-anal-cardiac-tracheoesophageal fistula-renal and limb)and heterotaxy.
Three cases had arrhythmia in the absence of structural malformations. Two of these resulted in hydrops and were considered causal, while in the third, the cause of death remained uncertain. Due to small numbers, these cases are not discussed further.
Cardiac weight was considered normal in an absolute sense if it fell between the 5th and the 95th centile for gestational age on the charts of Usher and McLean [1969. Using this definition, 142/940 (15.1%) of infants with recorded heart weight had absolute cardiomegaly, and 144/940 (15.4%) had absolute microcardia. Cardiac weight was considered abnormal in a relative sense if the gestational age based on heart weight was more than 2 weeks different from the gestational age based on total body weight using the tables of Usher and McLean [1969. Of the 940 cases with known heart weight, 224 (23.8%) had relative cardiomegaly, and 145 (15.4%) had relative microcardia. Relative and/or absolute cardiomegaly was found in 255 cases (27.1%) and increased with gestational age ranging from 9.1% at 13–16 weeks to 36.5% at >40 weeks (Fig. 3). Relative and/or absolute microcardia was found in 230 cases (24.5%) and showed no clear relationship to gestational age (Fig. 4).
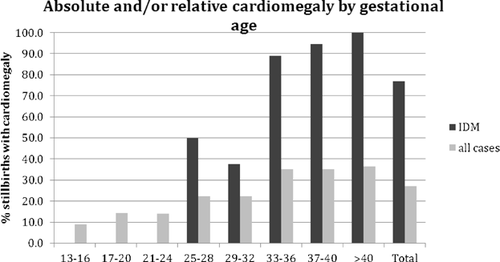

The most frequent association with cardiomegaly was maternal diabetes. Our cohort contained 68 infants with diabetic mothers (IDMs), of whom 39 had recorded heart weights. Absolute and/or relative cardiomegaly was found in 76.9% of all IDM's, 93% of those over 32 weeks, and 100% of those at 40 weeks (Fig. 3). Cardiomyopathy, sometimes with no recorded heart weight, was diagnosed in 26 cases, including 5 IDMs. Small heart size was not associated with cardiac malformations but was strongly associated with Turner syndrome. Among the 42 infants with Turner syndrome in the study, 11/14 with known heart weight and 4/4 with known heart-lung weight, all at <32 weeks, had absolute microcardia (Fig. 4).
DISCUSSION
The prevalence of CHD in our cohort of stillbirths (9.4%) is more than 10 times the ∼0.8% incidence found in the liveborn population. This indicates that CHD is a direct cause of fetal death (as evidenced by signs of fetal cardiac failure such as hydrops) and/or that it is a component of syndromes that cause fetal death. The latter appears most common since 70% of heart defects in our study are part of a lethal syndrome, while only 14.5% of the infants with heart defects (1.25% of the entire group of stillbirths with cardiac data) died from isolated CHD (Fig. 1). This reflects the fact that many heart defects become life-threatening only after birth when the infant must be able to independently maintain oxygenation. However, there were five infants whose only defect was premature closure of the ductus and/or foramen ovale. Premature closure of the ductus has been reported to cause fetal right heart failure [Hofstadler et al., 1996; Enzensberger et al., 2012] and was considered causal. Premature closure of the foramen ovale also occurs in association with fetal heart failure [Coulson and Kuller, 1994] but could be a consequence rather than a cause of fetal circulatory disturbance. The single case with isolated premature closure of the foramen ovale was included in the incidental group, which included 15.6% of heart defects (representing 1.35% of the entire cohort). Since the prevalence of incidental cardiac malformations in our stillborn cohort exceeds the prevalence of heart defects in the liveborn population, we need to consider the possibility that some of the defects classified as incidental may have contributed to fetal death. Alternatively, we may be detecting incidental anomalies in stillbirths that would have remained asymptomatic and resolved without ever being recognized if the infant had survived. The findings of Ishikawa et al. [2011 who found asymptomatic cardiac defects not requiring treatment in nearly 3% of unselected newborns who underwent echocardiography supports this interpretation.
While the prevalence of cardiac malformations among stillbirths in our study is slightly higher than the 7.9% among stillbirths found by Hoffman and Christianson [1978 and also in the 20–24-week group of Gerlis [1985, the prevalence of cardiac malformations in miscarriages <20 weeks in our study is considerably less than observed by Gerlis, who found an inverse relationship between CHD incidence and gestational age. They attributed the very high numbers of cardiac defects in the youngest embryos to the high incidence of chromosome abnormalities in first trimester losses, which were not included in our cohort. Furthermore, our study relied on routine pathology examination, which may miss malformations in very small hearts, while Gerlis [1985 was able to detect anomalies in tiny hearts by examination of very thin sections under high magnification. Thus, the incidence of CHD in our cohort (8.4%) should be considered a minimum, particularly in the younger fetuses.
All specific cardiac lesions for which comparable data exist were more frequent in our stillborn cohort compared to the recent data from the Center for Disease Control (CDC) [Bjornard et al., 2013](Table III). This CDC study was chosen for comparison because it is a recent large study which falls well within the ranges reported in previous studies as reviewed by Hoffman and Kaplan [2002. While it is expected that lesions with a high rate of prenatal lethality, such as ectopia cordis or Ebstein anomaly, would be more common in the stillborn cohort, it is noteworthy that even lesions not expected to have physiologic consequences in the fetus, such as ASD or a small VSD are more prevalent in stillbirths. This is of course partly attributable to syndromes (such as trisomy 18) that are lethal prenatally, even when the associated cardiac lesions are minor. Excluding defects that are part of syndromes, we would expect that the prevalence of non-lethal heart defects in stillborn infants would be comparable to that in livebirths, unless the cardiac lesion is contributing to the risk of stillbirth. For isolated VSD, the frequency in our stillborn cohort was only moderately increased compared to estimates in livebirths (6.2/1,000 vs. 2.2/1,000); but for isolated ASD, there is a 10-fold increase in the stillborn compared to the liveborn population (6.7/1,000 vs. 0.6/1,000). Because the foramen ovale is normally patent in the fetus, it is extremely unlikely that isolated ASD would compromise fetal cardiac function, but it is possible that a few of the ASDs might be markers for a syndrome that was overlooked in a small or macerated infant. It is also possible that a pathologist might mistake a widely patent foramen ovale for an ASD, although, in 78% of the cases with ASD, the pathologist provided clear evidence that the ASD was either completely separate from the foramen ovale or was much too large to represent simply a patent foramen ovale. The most likely explanation is that the frequency of ASD identified in living children is lower due to spontaneous closure that can occur prenatally or postnatally as well as missed cases in asymptomatic children who never have cardiology evaluation. Ishikawa et al. [2011 found large ASDs that did not close neonatally in 8/2,067 echocardiograms of consecutive newborns, for an incidence of 3.9/1,000, suggesting that without echocardiography, ASDs in neonates have been underestimated. In the same study, VSDs were found in 26.1/1,000, which is significantly higher than isolated VSDs in our study (6.2/1,000). This suggests that small muscular VSDs, although recognizable on ultrasound, are missed in current autopsy protocols, and in fact, of the 54 cases with VSD, 35 were described as membranous, 18 had vague descriptions such as “large VSD,” and only one was apical. Alternatively, since the incidence of VSD in these livebirths without long term follow-up is similar to our observation of VSD in 25.9/1,000 stillbirths overall, including those with identifiable syndromes, it is possible that non-lethal syndromes may be overlooked in studies of neonates, especially if there is no long-term follow-up of growth and development.
In our stillborn cohort, most CHD (70.2%) was associated with other anomalies. While recognizing that these actually comprise a variety of syndromes, sequences, and associations, for convenience we refer to this group as “syndromic” (Fig. 2). In the group with multiple anomalies, almost half (57/130 = 44%) had a chromosomal syndrome, most frequently trisomy 21 (18) and trisomy 18 (16), followed by various structural anomalies (7), trisomy 13 (6), Turner syndrome(5), and diGeorge sequence (3), mosaic rare aneusomies (2), and triploidy (1). In cases with unsuccessful chromosome studies before the availability of fluorescence in situ hybridization (FISH), clinical diagnoses were made when the evidence was strong (including DiGeorge in the presence of conotruncal heart defects and thymic aplasia as well as Down syndrome in the presence of typical facial, hand, and radiologic features). Ectopia cordis occurred in 8%, all of which had other anomalies including limb-body wall complex (3), amniotic bands (3), omphalocele, exstrophy of the cloaca, imperforate anus, and spinal malformation (OEIS) (3), and in one case an undefined schisis association. An additional infant, counted in the “other” group, had ectopia cordis as a feature of short rib polydactyly. Three fetuses (2%) had tumors involving the heart, and another, counted in the chromosomal category due to microdel 12q, had an incidental cardiac fibroma. Among the 55/129 (45%) who were placed in an “other” category, recurring specific diagnoses were vertebral-anal-cardiac-tracheoesophageal fistula-renal and limb (VACTERL) association (6), heterotaxy (5), diabetic embryopathy (4), and Noonan syndrome (2). Also included in the “other” group were nine with various renal anomalies and five with various skeletal dysplasias in addition to cardiac anomalies. There were 12 with other known conditions and 12 with multiple congenital anomalies that could not be further categorized. Four infants with lethal non-cardiac conditions (anencephaly, osteogenesis imperfecta, and two renal agenesis cases) had incidental ASD found at autopsy.
While chromosomal abnormalities are frequently associated with CHD [Roskes et al., 1990; Pierpont et al., 2007; Hartman et al., 2011], the incidence of CHD associated with chromosomal abnormalities in our study was much higher than in liveborn infants. For example, Hartman et al. [2011 found the incidence of chromosomal abnormalities among infants with CHD to be 12.3%, while our stillbirth sample had an incidence of 33%. Most (70%) of the chromosomal abnormalities were trisomies (Fig. 1), a finding similar to those of liveborn studies, but the ratio of trisomy 18 to trisomy 21 was shifted from approximately 1:8 in Hartman's study to nearly 1:1 in our stillborn group. Since virtually all liveborns with trisomy 18 have congenital heart disease, compared to only about half of those with Down syndrome, the higher prevalence of trisomy 18 in stillbirths accounts for much of the difference. Microdeletions such as deletion 22q11 were presumably underdiagnosed, as very few of the stillborn infants had FISH or chromosomal microarray.
In addition to CHD, cardiomyopathy and heart size were also investigated as potential contributors to stillbirth. Cardiomyopathy is quite rare in the liveborn population, with rates ranging from about 4/100,000 to 8/100,000 [Arola et al., 1997; Lipshultz et al., 2003; Nugent et al., 2003]. However, it is well established that the incidence of hypertrophic cardiomyopathy is much higher in IDMs, with rates as high as 32% [Gutgesell et al., 1980] to 38% [Abu-Sulaiman and Subaih, 2004], and that cardiomyopathy/cardiomegaly in IDMs can contribute to fetal death. Cardiomyopathy, either dilated or hypertrophic, was diagnosed at autopsy in 26/2,083 cases in our study, 20% of which were born to diabetic mothers, even though only 3% of our cases were IDMs. Hypertrophic cardiomyopathy is diagnosed in living infants by echocardiographic measurements of septal and ventricular wall thickness, but cardiomegaly by weight, measured at autopsy, may function as a surrogate for recognition of cardiomyopathy in stillborn infants for whom septal and ventricular wall measurements are not available. Using the autopsy-based data of Maroun and Graem [2005, we found heart weight above the 95th centile in 15.1%, which is more than expected, suggesting that compared to other perinatal autopsies stillborn infants are more likely to have large hearts. Relative cardiomegaly (heart size more than 2 weeks ahead of body weight) was even more common, occurring in 23.4% of our cohort, but no standards are established. Cardiomegaly, both relative and absolute, increased with gestational age from 9% at 13–16 weeks to 36% at term in our cohort (Fig. 3). Much of this increase was due to a very high prevalence of cardiomegaly among IDMs, especially at later gestational ages. Of the 67 IDMs in our study, 76.9% had either relative or absolute cardiomegaly, with the incidence tending to increase with gestational age (Fig. 3). Indeed, nearly 95% of IDMs in the 37–40-week-old range had cardiomegaly, and all IDMs at or above 40 weeks gestational age had both relative and absolute cardiomegaly (Fig. 3). Maternal diabetes may contribute to cardiomegaly through maternal hyperglycemia leading to fetal hyperinsulinemia and increased fetal growth. The combination of hyperglycemia and fetal hyperinsulinemia may also affect the placental vasculature [Leach, 2011]. Increases in placental perfusion impedance have been shown to alter the size of the fetal heart, as the heart works to maintain its stroke volume to provide blood to the developing body [Thornburg et al., 2010]. These changes are likely time dependent due both to worsening of gestational diabetes and cumulative effects of exposure to the maternal diabetic mileu. The excess of cardiomegaly in cases without known maternal diabetes could reflect underdiagnosis of maternal diabetes, other conditions affecting placental vasculature, and intrinsic fetal causes of hypertrophic cardiomyopathy.
Small heart size was also more frequent than expected, with 15.3% of our stillborn cohort having heart weights below the 5th centile of Maroun and Graem [2005 (Fig. 4). Over half (78/144 = 54%) of the fetuses with absolute microcardia were SGA. Although this suggests that at least some causes of intrauterine growth retardation may affect the heart proportionately, the observation of relative cardiomegaly in 26% of the SGA fetuses (which is comparable to the prevalence of relative macrocardia in the entire cohort) suggests that a significant proportion of SGA fetuses have normal sized hearts. This may reflect various mechanisms for fetal growth retardation that affect the heart differently, for example, placental vascular disease might increase the cardiac workload. It is likely that overestimation of gestational age in small fetuses with prolonged intrauterine retention might contribute to absolute microcarda (small fetuses with proportionately small hearts). If genes affecting cardiac development also cause cardiac hypoplasia, the group with structural cardiac malformations might be expected to have smaller hearts, but in fact the prevalence of microcardia in fetuses with structural cardiac malformations and known heart weight is comparable to that for the entire cohort (10/73 = 13.7%) with absolute microcardia. Numbers are too small to determine if specific cardiac lesions such as hypoplastic left heart are associated with small heart size. Relative microcardia with heart size lagging more than 2 weeks behind body weight was observed in 15.4%. This might reflect conditions such as hydrops that can increase body weight without growth of internal organs, and in fact 25/69 (36%) infants with hydrops and known heart weight had relatively small hearts which is significantly more than expected. Most of the excess, however, was due to Turner syndrome. There were 42 cases of Turner syndrome in the entire cohort, of which only 14 had recorded heart weights. Absolute (11/14 = 78.6%) and relative (9/14 = 64.3%) microcardia were frequent (Fig. 3), supporting the opinion of Barr and Oman-Ganes [2002 that cardiac hypoplasia may be a common feature of Turner syndrome contributing to the high prenatal mortality(Fig. 4).
CONCLUSIONS
Congenital heart disease is about 10 times more frequent in stillbirths than in liveborn neonates. While all cardiac anomalies for which comparable data exist are more frequent in stillbirths, the most frequent categories in stillbirths are severe cyanotic lesions, VSD, and ASD. Most of the increase is due to congenital heart disease occurring as part of lethal syndromes, about one-third of which are chromosomal. Although isolated lethal CHD was recognized in only 1% of the stillborn cohort, the observation that fewer cases were recognized in younger fetuses raises concern that current autopsy protocols, which frequently include only external examination of the smaller hearts, may miss cardiac anomalies. Incidental cardiac defects were found about twice as often as expected, suggesting that a significant number of incidental defects may resolve prenatally or remain undiagnosed in liveborn infants.
Cardiomegaly or cardiomyopathy on ultrasound is common in fetuses of diabetic mothers approaching term and may be a valuable clue to identification of infants at risk for stillbirth who could benefit from early delivery. At the other extreme, cardiac hypoplasia is common in stillbirths with Turner syndrome even in the absence of cardiac malformation and may contribute to the very high fetal mortality.
In summary, cardiac evaluation of stillborn infants, including cardiac weight as well as dissection of the heart to identify internal malformations, is a vital part of the etiologic evaluation of stillbirth. Evaluation for fetal chromosomal disorders is important in all stillbirths and whenever cardiac malformations are identified prenatally. Lesions that are not usually detected in livebirths, such as premature closure of the ductus and/or foramen ovale, need to be studied in more detail to learn if they contribute to perinatal mortality. Cardiomegaly, if recognized prenatally, may be an indication of diabetic cardiomyopathy in the fetus, while small heart may be a marker for Turner syndrome and may help to explain the high fetal mortality for that condition. More work is needed to determine if estimation of cardiac weight can be an indicator of impending fetal demise and the need for urgent delivery in diabetic mothers during the third trimester.
ACKNOWLEDGMENTS
We would like to thank the parents and families who have suffered a stillbirth or miscarriage and are included in the WiSSP database. We also acknowledge the providers throughout Wisconsin and elsewhere who refer their patients to our program. We also would like to thank the Office of Scientific Writing and Publications at the Marshfield Clinic Research Foundation for editorial assistance with this manuscript.




