Dysspondyloenchondromatosis without COL2A1 mutation: Possible genetic heterogeneity
Abstract
Dysspondyloenchondromatosis is a rare form of generalized enchondromatosis associated with spinal involvement. This skeletal dysplasia is characterized by multiple enchondromas present in vertebrae as well as in metaphyseal and diaphyseal parts of the long tubular bones, post-natal short stature, and early development of kyphoscoliosis. A novel heterozygous missense mutation in COL2A1 was recently identified in a patient with dysspondyloenchondromatosis. This suggests that dysspondyloenchondromatosis might expand the already broad spectrum of type II collagenopathies. Here, we report on a young girl with features of dysspondyloenchondromatosis, specifically short stature, thoracoscoliosis, and generalized enchondromas lesions. Sanger sequencing failed to detect a mutation in COL2A1. We therefore suggest that dysspondyloenchondromatosis is a genetically heterogeneous condition. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Dysspondyloenchondromatosis (DSC or spondyloenchondromatosis) is a rare form of generalized enchondromatosis associated with spinal involvement. This entity differs from other forms of enchondromatosis by the severe spinal deformities mimicking vertebral malsegmentation [Pansuriya et al., 2010]. Dysspondyloenchondromatosis belongs to the group of spondylometaphyseal dysplasias [Warman et al., 2011]. It was initially described by Freisinger et al. [1993] and subsequently four additional patients were reported [Kozlowski et al., 1994]. This disease is characterized by post-natal short stature and multiple enchondromas in the vertebral bodies and the metaphyseal or diaphyseal parts of the long tubular bones. These enchondromas lead to severe and early-onset kyphoscoliosis, and unequal limb length. In patients with DSC, the dysplastic process usually affects one or more adjacent vertebral bodies to the extent that severe structural changes simulate a segmentation anomaly [Freisinger et al., 1993; Kozlowski et al., 1994]. To the best of our knowledge, 14 patients have been reported in the literature [Mainzer et al., 1971; Spranger et al., 1978; Azouz, 1987; Lerman-Sagie et al., 1987; Freisinger et al., 1993; Kozlowski et al., 1994; Haga et al., 1998; Nakane et al., 2011; Kenis et al., 2013]. Recently Nakane et al. [2011] described a boy with DSC with a novel heterozygous missense mutation in the COL2A1 gene, leading to the substitution of a bulky amino acid by a glycine.
In this paper, we report on a young girl with clinical and radiological features of DSC with no mutation in the coding sequences of COL2A1.
CLINICAL REPORT
The patient was the only child of unrelated healthy parents originating from France. She was born at 34 weeks of gestation after an uneventful pregnancy. Birth parameters were in the normal range (length 44 cm, −2.9 SD; weight 2,475 g, −0.3 SD; OFC 35 cm, +1.6 SD). She was referred to the Genetic Department for severe post-natal short stature (length 79.5 cm, −4 SD at 3 years and 4 months). Her motor, language and intellectual development was reported to be normal, but was not formally evaluated. She had hip claudication diagnosed at the age of two due to transient synovitis.
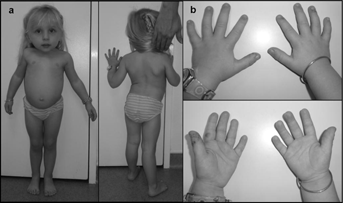
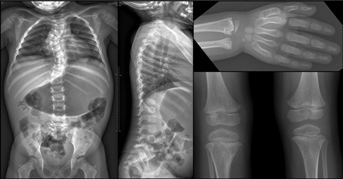
At the most recent examination, at four years of age, her height was 84.5 cm ( 4 SD); weight was 11.5 kg (−2.5 SD); and OFC was 53 cm (+2.5 SD). She presented with a short thorax, pectus carinatum, and severe thoracoscoliosis associated with hyperlordosis (Fig. 1a and b). Examination of joints showed elbow limitation. Urinary organic acid chromatography was also normal excluding metaphyseal enchondromatosis with 2-hydroxyglutaric aciduria (MC-HGA, OMIM 271550). Abdominal, renal and heart ultrasounds were also normal. Skeletal X-rays showed spondylometaphyseal dysplasia with generalized enchondromatosis. On the vertebral column, there was spinal involvement with severe thoracic scoliosis and hyperlordosis associated with enchondromatosis of vertebral bodies mimicking vertebral malsegmentation. Vertebral enchondromas were confined to the thoracic and lumbar spine. Less severe enchondromas were also observed both on distal radius and ulna and on proximal tibia (Fig. 2). In addition, no asymmetry of the limbs was observed.
Genetic Analysis
Genomic DNA was isolated from peripheral EDTA anti-coagulated whole blood samples of the patient and her healthy parents using QIAamp DNA Blood Midi Kit (QIAGEN, Courtaboeuf, France) according to the supplier's protocol. PCR amplifications of the 54 exons and the intron–exon junctions of COL2A1 gene (NG.008072.1–NM_001844.4) were performed using two pairs of primers (sequences available upon request), using the Promega PCR Master Mix, according to the manufacturer's protocol (Promega, Madison, WI). Mutation screening was performed by direct bi-directional sequencing, using the BigDye terminator cycle sequencing kit (BDT v3.1), followed by electrophoresis of the amplicons on an ABI 3100XL Genetic Analyzer, according to the manufacturer's recommendations (Applied Biosystems, Foster City, CA). The obtained sequences were aligned to the reference DNA sequence of the COL2A1 gene (NG_008072.1) using the Seqscape 2.6 Software (Applied Biosystems). DNA sequence information referred to the public UCSC database GRCh37 (hg19).
We identified several nucleotide variations, among which a variant of unknown significance (VoUS), c.610-7G>A in intron 8. The proband inherited this heterozygous VoUS from her healthy mother. The patient's karyotype was normal (46,XX).
DISCUSSION
Skeletal dysplasia with enchondromatosis manifests both phenotypic and genetic heterogeneity. The first classification of enchodromatosis was proposed by Spranger et al. [1978]. The authors classified enchondromatosis with irregular vertebral lesions as type V, later coined DSC [Pansuriya et al., 2010]. More recently, DSC was classified as type VII enchondromatosis by Superti-Furga et al. [2012]. Among the subtypes of generalized enchondromatosis with spinal involvement, three distinct entities are described: (1) spondylenchondrodysplasia (SPENCD, OMIM 271550) characterized by intellectual disability, cerebral calcification, autoimmunity and mild to moderate vertebral involvement, which is due to mutations in ACP5 [Superti-Furga et al., 2012]; (2) metaphyseal enchondromatosis with 2-hydroxyglutaric aciduria (MC-HGA, OMIM 271550) characterized by intellectual deficiency, vertebro-metaphyseal involvement and hydroxyglutaric aciduria, which is due to mutations in IDH1 or IDH2 [Superti-Furga et al., 2012] and (3) DSC. Dysspondyloenchondromatosis is characterized by enchondromatosis and severe involvement of vertebrae leading to radiological aspects similar to those observed in vertebral segmentation anomaly.
The clinical and radiological features of the 14 previously reported patients as the present patient are summarized in Table I. Most patients were born at term. Their birth lengths were all below 50 cm, ranging from 39.5 cm (−6 SD) to 49 cm (mean) with an average at 43.4 cm (−4 SD). Length or height at last examination ranged from −3 to −9.8 SD (median, −5.6 SD), suggesting that severe short stature is a component of this disorder. The severe short stature could in part be due to the severe and frequent scoliosis. Indeed, all patients presented with scoliosis except patient 3 described by Kozlowski et al. [1994]. A second element that might be responsible for this severe short stature is the limb asymmetry. All patients, except for the patient reported here, presented with limb asymmetry detected in the first years of life. Asymmetric limbs might be the result of asymmetric localization on femoral and tibial enchondroma growth plates that could lead to limb length inequality. The patient reported here did not have major femoral or tibial enchondromas that could explain the absence of limb asymmetry.
| Mainzer et al. [1971] | Spranger et al. [1978] | Azouz [1987] | Lerman-Sagie et al. [1987] | Freisinger et al. [1993] | Kozlowski et al. [1994] | Haga et al. [1998] | Nakane et al. [2011] | Kenis et al. [2013] | Present report | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 1 | Patient 2 | Patient 3 | Patient 4 | |||||||||
| Gender | F | M | M | F | M | M | F | M | M | F | M | F | M | M | F |
| BW, g | NR | 2,400 | 2,500 | 2,450 | 2,950 | 3,450 | 2,850 | 3,045 | 3,500 | 2,100 | 3,650 | 3,060 | 2648 | NR | 2,475 |
| BL, cm (SD) | NR | 40 (−6) | NR | NR | 40.5 (−5.5) | 43 (−4) | 41 (−5) | 46 (−2) | 47.7 (−1.5) | 39.5 (−6) | 46 (−2) | 49 (Me) | 41 (−5) | NR (−3) | 44 (−2.5) |
| Age at diagnosis | 18 months | 4 years | 3 years | 2 years and 7 months | 3 years and 6 months | 13 months | 2 years and 6 months | 1st day | 1st day | 4th day | 5th day | 1 year | 3 y | 5 years | 5 years |
| Age at last examination | 20 years | 4 years | 3 years | 2 years and 7 months | 3 years and 6 months | 13 months | 17 months | 5 years | 4 years and 6 months | 15 months | 3 years and 9 months | 7 years | 14 y | 5 years | 5 years |
| Weight, kg (SD) | NR | NR | NR | 11.25 (−1.2) | 9 (−4.5) | 8,2 (−1.7) | 7.5 (−3) | NR | NR | NR | 10, (−3) | NR | 21 (−3, 4) | NR | 11.5 (−2, 5) |
| Height, cm (SD) | NR | 82.5 (−6) | NR | 80 (−3) | 69 (−6) | 63 (−5) | 63.5 (−5.5) | NR | NR | NR | 72 (−6) | NR | 103 (−9, 8) | NR | 84.5 (−4) |
| OFC, cm (SD) | NR | 51.2 (+1.2) | NR | Me | 51 (+0.5) | NR | 48 (Me) | NR | NR | NR | NR | NR | NR | NR | 53 (+2, 5) |
| Intellectual developmenta | Above average | Normal | NR | Delayed | NR | NR | Normal | NR | Normal | Delayed | Normal | NR | Normal | Normal | Normal |
| Asymmetric limbs (detected age) | + At birth | +4 years | +3 years | +2 years and 7 months | +At birth | +13 months | +17 months | +At birth | +2 years and 6 months | +At birth | +5 weeks | +1 year | +3 years | +5 years | − |
| Vertebral malsegmentation or hypodysplasia | + | ++ | +++ | + | ++ | ++ | +++ | ++ | ++ | ++ | ++ | + | + | +++ | + |
| Scoliosis | +++ | ++ | +++ | +++ | +++ | ++ | ++ | + | ++ | None at 15 months | +++ | +++ | +++ | ++ | +++ |
| Enchondromata | + | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | +++ | + | ++ | ++ | + |
- BL, birth length; BW, birth weight; F, female; M, male; Me, mean; m, months; y, years; NR, not reported; SD, standard deviation; y, years.
- +, mild; ++, moderate; +++, severe involvement.
- a Claims for intellectual development are estimates, as formal testing was not performed.
A patient with DSC was recently reported with a novel heterozygous missense mutation in COL2A1. It was an apparently de novo heterozygous c.2258G>A (p.Gly753Asp) mutation in exon 34. It suggests that DSC might expand the broadspectrum of type II collagenopathies [Nishimura et al., 2005; Nakane et al., 2011].
Type II collagenopathies result from mutations in the COL2A1 gene, which are responsible for several skeletal dysplasias [Spranger et al., 1994; Warman et al., 2011]. In addition to the classical skeletal anomalies observed in Type II collagenopathies Walter et al. [2007] described four individuals with COL2A1 mutation and metaphyseal involvement with enchondroma-like lesions extending from the metaphysis to the diaphysis.
However, the patient reported here carries several frequent COL2A1 SNPs and a maternally inherited VoUS, c.610-7G>A, with no clear pathogenic mutation. Moreover, we have identified this VoUS in other patients with type 2 collagenopathies, but it was often inherited through asymptomatic parents.
We report a girl who fulfills the DSC criteria namely short stature, scoliosis, enchondromas, vertebral involvement, and apparently normal intellectual development and we show that she did not have mutation in COL2A1. We propose that patients with DSC should be screened for COL2A1 mutations, to determine if DSC is a genetically heterogeneous condition.
ACKNOWLEDGMENT
We thank the patient and her family.




