Patient with three euchromatic supernumerary marker chromosomes derived from chromosomes 1, 12, and 18: Characterization and evaluation of the aberrations
Abstract
The genetic relevance of small supernumerary marker chromosomes (sSMCs) depends on their content of euchromatin. In case of mosaicism, the phenotype of the carrier furthermore is influenced by the distribution of the marker in the body. In the majority of reported cases no correlation of the degree of mosaicism in the tissue(s) analyzed and the phenotype could be detected. In particular, non-acrocentric derived sSMCs show a strong tendency to appear in mosaic state irrespective of the clinical picture. We present a patient with cognitive disability and mild craniofacial dysmorphisms with mosaicism of three different autosomal marker chromosomes. The extra chromosomes were analyzed by a combination of SNP array and a variety of fluorescence in situ hybridization (FISH) probes. All three markers were identified as ring chromosomes containing different amounts of euchromatic material derived from chromosome 1 (1p12 → q21), 12 (12p13.1 → q13.11) and 18 (18p11.21 → q11.2). The size and the frequency of the sSMCs were strikingly different, besides, we observed an unequal combination of the three derivates. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Small supernumerary marker chromosomes (sSMCs) are a heterogeneous group of derivatives which cannot be characterized unambiguously by conventional cytogenetic techniques. sSMCs are detected in 0.04% of live births [Liehr and Weise, 2007; for review Hu et al., 2011]. The probability for an abnormal phenotype is approximately 13%, and depends on its chromosomal origin and its formation (de novo or inherited) [Liehr and Weise, 2007; Manolakos et al., 2010].
The variable composition of marker chromosomes and diverse content of relevant genetic material pose a great difficulty for the prediction of the outcome and implications. Therefore, the precise identification of the genetic structure is essential for genotype-phenotype correlation [Starke et al., 2003]. Heterochromatic marker chromosomes are not suspected to result in phenotypic abnormalities [Liehr and Weise, 2007]. The clinical course is furthermore influenced by the distribution of mosaicism. However, in sSMC cases with an abnormal phenotype and mosaicism, the clinical outcome does usually not depend on the ratio of aberrant cells in the analyzed tissue.
The standard procedure to characterize marker chromosomes combines cytogenetic and molecular techniques, for example, sSMCs can be successfully identified by a combined FISH analysis with both whole chromosome painting probes (wcp-FISH) and centromere specific probes (cen-FISH). Centromere probes additionally verify the number of centromeres, as marker chromosomes are often unstable and have a dicentric structure. The second relevant technique for the identification of marker chromosomes is molecular karyotyping which allows the analysis of its breakpoints as well as the detection of mosaic levels higher than 10%.
Here, we report on a 4-year-old girl with dysmorphisms and developmental delay associated with an unusual mosaic karyotype exhibiting three ring marker chromosomes originating from chromosomes 1, 12, and 18.
CLINICAL REPORT
The patient is a 4-year-old Iranian girl. The parents were healthy and non-consanguineous. Pregnancy and delivery were uneventful. Birth measurements were within the normal range [length 53 cm (50th–75th centile), weight 3,600 g (50th–75th centile), head circumference 36 cm (75th centile)]. She was referred to clinical examination because of a moderate developmental delay at the age of 4 years. She did not speak single words before 18 months and developed no further speech capacities. She was able to sit at the age of 4, at the same age she learned to walk alone. Formal intelligence testing was not undertaken. Her height, weight, and head circumference were still within the normal range and no inner organ malformations were detected. She presents only mild craniofacial and limb dysmorphisms (Table I, Fig. 1). Her phenotype was in good accordance with findings in duplication 12p [Izumi et al., 2012] and is mainly characterized by facial dysmorphisms combined with cognitive disability (Table I). About 24 genes are localized in the duplicated region 12p of our patient, but a specific correlation of single genes with the clinical symptoms of the patient could not be delineated.
| Clinical features of partial duplication 12p | Our patient |
|---|---|
| Prominent forehead | − |
| Partial alopecia | − |
| Bushy eyebrows | + |
| Hypertelorism | + |
| Epicanthus | + |
| Exophthalmus | − |
| Small nose | + |
| Anteverted nares | + |
| Broad nasal bridge | + |
| Full cheeks | + |
| Poorly modelled philtrum | + |
| Large mouth | + |
| Everted lower lips | + |
| Downslanted corners of the mouth | + |
| Small chin | + |
| Low-set ears with overfolded helix | + |
| Clinodactyly and brachyphalangy V | + |
| Cognitive disability | Moderate |
| Seizures | − |
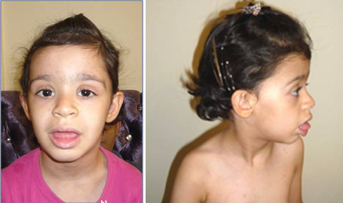
The phenotype of Pallister–Kilian syndrome was not compared to the clinical findings in our patient as it is based on a triplication and besides includes the total short arm of chromosome 12. Our patient shows fewer clinical symptoms than listed in the literature and the cognitive disability of the girl was documented as moderate. This difference might be caused by the mosaic status of the aberration and/or by the fact that the duplicated region includes only about 60% of the total length of 12p. The duplicated region of 12q11 to q13.1 seems to induce no specific phenotypical changes.
Duplications of the regions 1p and 18p reveal only nonspecific alterations like epicanthic fold, low-set dysmorphic ears, and a small chin.
CYTOGENETIC AND MOLECULAR GENETIC INVESTIGATIONS
Conventional chromosomal analysis from peripheral lymphocytes by standard GTG banding resulted in a mosaic karyotype with four different cell lines: mos48,XX, +mar1, +mar2[31]/47,XX, +mar1[22]/49,XX, +mar1, +mar2, +mar3[3]/46,XX[5]. Parental karyotypes were normal.
SNP array investigation (Affymetrix 6.0 SNP array) revealed gains in the euchromatic chromosomal regions 1p12q21, 12p13.1q13.11 and 18p11.2 (Fig. 2) (ISCN-based array karyotypes: (NCBI36/hg18): arr1p12p11.2(117,679,636–121,045,604) × 3, 12p13.1q13.11(141,521,68–450,000,00) × 3, 18p11.21p11.21(121,353,99–151,33714) × 3).
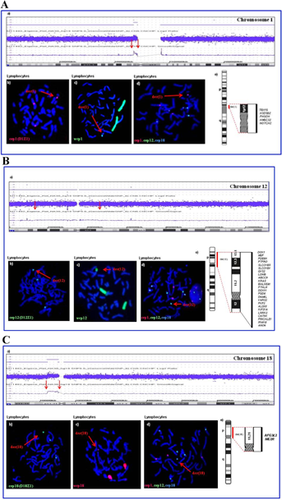
The chromosomal origin of the three marker chromosomes was confirmed by FISH analyses using combinations of centromeric and whole chromosome painting DNA probes of chromosomes 1, 12, and 18 (Fig. 2). Additionally, the distribution and frequencies of the three derivative chromosomes were analyzed in both lymphocytes (metaphases and interphases) and buccal mucosa cells by FISH (Table II). In lymphocytes the frequency of marker positive cells was higher in metaphases than in interphases. Generally, an unequal distribution of the marker chromosomes could be observed, with (slight) differences between the two different cell systems. Comparing interphases of lymphocytes and buccal mucosa, the amount of marker positive cells was higher in lymphocytes. Less than 4% of analyzed cells showed all three derivatives, the majority of aberrant cells carried at least the der(18). None of the three marker chromosomes showed changes in size (duplication) or number per cell in the cell systems investigated.
| Type of segregation | Lymphocytes number of cells (frequency in %) | Buccal mucosa cells number of cells (frequency in %) |
|---|---|---|
| 47,XX, +der(1)a | 27 (12.7) | 27 (12.5) |
| 47,XX, +der(12)a | 5 (2.4) | 20 (9.3) |
| 47,XX,der(18)a | 60 (28.3) | 59 (27.3) |
| 48,XX, +der(1), +der(12) | 5 (2.4) | 5 (2.3) |
| 48,XX, +der(12), +der(18) | 15 (7.0) | 6 (2.8) |
| 48,XX, +der(1), +der(18) | 33 (15.6) | 17 (7.8) |
| 49,XX, +der(1), +der(12), +der(18) | 6 (2.8) | 8 (3.7) |
| 46,XX | 61 (28.8) | 74 (34.3) |
| Total number of cells | 212 | 216 |
| With der(1) | 71 (33.5) | 57 (26.4) |
| With der(12) | 31 (14.6) | 39 (18.1) |
| With der(18) | 114 (53.8) | 90 (41.6) |
- a Frequency of the three different marker chromosomes in the four pathological cell lines.
By microsatellite typing, the paternal origin of the chromosome 1 and 18 derivatives could be determined. The derivate 12 could not be further delineated because of its low frequency.
RESULTS AND DISCUSSION
The simultaneous occurrence of three different marker chromosomes in seven aberrant cell lines of the same individual is an extremely rare finding in postnatal cytogenetic diagnostics and makes a comprehensive laboratory workup necessary. We report on a girl with developmental delay carrying a complex mosaic karyotype which was initially diagnosed by conventional GTG banding. The chromosomal origins and sizes of the different markers were delineated by SNP array typing. By FISH analyses, the chromosomal origin could be corroborated and the distribution in two cell systems—lymphocytes and buccal mucosa cells—was determined (Table II).
The der(1)(1p12q21) marker shows an euchromatic size of 3.3 Mb and includes 5 disease causing genes (Fig. 2A-a). By interphase FISH investigation it could be detected in 71 out of 212 lymphocytes (33.5%) and in 57 out of 216 buccal mucosa cells (26.4%) (Table II). More than two-thirds of the reported der(1) cases are associated with clinical abnormalities. Indeed, Liehr et al. [2010] reported a case with a chromosome 1 derivative of similar size and shape without any malformations. In addition, by DA/DAPI staining, the der(1) could be shown to consist mainly of heterochromatin (p12q21) (Fig. 2A-b,c). ***Therefore we assume that this marker does not influence the phenotype in our patient to a larger extent.
The der(12)(12p13.1q13.11) has a size of 30.8 Mb and harbors 24 OMIM disease causing genes (Fig. 2B-a). FISH investigation in peripheral blood and buccal mucosa cells revealed that the der(12) is the rarest marker (31 out of 212 lymphocytes (14.6%), 39 out of 216 in buccal mucosa cells (18.1%)). This relatively low degree of mosaicism was confirmed by SNP array typing (Fig. 2B-a). The der(12) showed an unequal distribution in the two cell systems, a finding which might be explained by the instable nature of der(12)s reported in the literature [Schubert et al., 1997]. Der(12) showed a larger signal with the whole chromosome painting probe than with the centromere probe which further proves the existence of euchromatin harboring transcriptionally active genes. The der(12) in our patient influences the patient's phenotype (Table I) as she shows many features of the craniofacial phenotype of patients with dup(12p)syndrome also described by Izumi et al. [2012].
The der(18)(p11.21q11.2) is the smallest of the three markers, it contains 3 Mb of euchromatin and includes only two OMIM disease causing genes (Fig. 2C). This derivative was the most frequent one in the patient. It could be detected by FISH in 114 out of 212 analysed lymphocytes (53.8%) and 90 out of 216 buccal mucosa cells (41.6%). Starke et al. [2003] reported on a patient carrying a similar shaped and sized derivative with the same breakpoints ascertained due to primary sterility. In the same publication a further case, a prenatally identified foetus with hygroma colli was reported carrying a der(18)(p11.1q11.1). In our case, the centromere FISH probe covers almost the whole length of the marker chromosome supporting the SNP array results of preponderance of heterochromatin (Fig. 2C-b,c).
By conventional chromosome staining and FISH, it was shown that the der(1) and the der(18) mainly consist of heterochromatin. If this is taken into account and the total length of duplication is delineated according to the structure in the ISCN ideogram the der(1) comprises 50%, der(12) 35% and der(18) 15% of heterochromatin compared to the total length of the respective marker. Therefore, the distal breakpoints in chromosome 1 and 18 have to be defined as 1q21 and 18q11.2. The three additional ring chromosomes are: r(1)(p12q21), r(12)(p13.1q13.11) and r(18)(p11.21q11.2).
The frequency of three derivatives in two different germ layers (lymphocytes from mesoderm and buccal mucosa cells from ectoderm) determined by molecular-cytogenetic analyses revealed a complex pattern of their mosaic distribution (Table II). In two of the derivatives, der(1) and der(18), the paternal origin of the supernumerary material could be delineated. All analysed markers showed reduction of the paternal heterozygosity to homozygosity. These observations indicate a prezygotic origin of the rearrangements and a postzygotic development of the mosaicism. This is in accordance with the recently published report of Robberecht et al. [2012] who hypothesized multiple prezygotic errors followed by a number of postzygotic trisomy rescue mechanisms as a reason for a mosaic karyotype including a normal cell line and cell lines with structural aberrations. We therefore assume that different rearrangements lead to a 49,XX, +mar1, +mar2, +mar3 zygote formation followed by postzygotic rescue mechanisms. The result is a complex mosaic karyotype consisting of eight different cell lines with unequal distribution of the three derivatives.
The influence of sSMCs on a patient's phenotype depends on factors like their content of euchromatin and the distribution of the mosaicism. The phenotype in our patient is in good accordance with the known chromosomal syndrome dup(12p). The FISH and array investigations revealed only small amounts of euchromatin in the der(1) and the der(18) in our patient. Thus, their influence on the patient's phenotype is minimal.




