Microarray and FISH-based genotype–phenotype analysis of 22 Japanese patients with Wolf–Hirschhorn syndrome
Abstract
Wolf–Hirschhorn syndrome (WHS) is a contiguous gene deletion syndrome of the distal 4p chromosome, characterized by craniofacial features, growth impairment, intellectual disability, and seizures. Although genotype–phenotype correlation studies have previously been published, several important issues remain to be elucidated including seizure severity. We present detailed clinical and molecular-cytogenetic findings from a microarray and fluorescence in situ hybridization (FISH)-based genotype–phenotype analysis of 22 Japanese WHS patients, the first large non-Western series. 4p deletions were terminal in 20 patients and interstitial in two, with deletion sizes ranging from 2.06 to 29.42 Mb. The new Wolf–Hirschhorn syndrome critical region (WHSCR2) was deleted in all cases, and duplication of other chromosomal regions occurred in four. Complex mosaicism was identified in two cases: two different 4p terminal deletions; a simple 4p terminal deletion and an unbalanced translocation with the same 4p breakpoint. Seizures began in infancy in 33% (2/6) of cases with small (<6 Mb) deletions and in 86% (12/14) of cases with larger deletions (>6 Mb). Status epilepticus occurred in 17% (1/6) with small deletions and in 87% (13/15) with larger deletions. Renal hypoplasia or dysplasia and structural ocular anomalies were more prevalent in those with larger deletions. A new susceptible region for seizure occurrence is suggested between 0.76 and 1.3 Mb from 4pter, encompassing CTBP1 and CPLX1, and distal to the previously-supposed candidate gene LETM1. The usefulness of bromide therapy for seizures and additional clinical features including hypercholesterolemia are also described. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Wolf–Hirschhorn syndrome (WHS, OMIM#194190), first described independently by Hirschhorn et al. [1965] and Wolf et al. [1965], is a contiguous gene deletion syndrome of the distal 4p chromosome characterized by a distinctive facial appearance, pre- and postnatal growth impairment, intellectual disability, and seizures [Battaglia et al., 2008]. In recent years, chromosomal microarray-based analysis has enabled us to identify WHS patients harboring well-defined variable-sized 4p deletions with or without additional duplications of other chromosomal regions as a result of unbalanced derivatives determined by concurrent metaphase fluorescence in situ hybridization (FISH) analysis [South et al., 2008c; Zollino et al., 2008].
Detailed genotype–phenotype correlation studies have also been performed using FISH analysis [Battaglia et al., 1999; Zollino et al., 2000] and chromosomal microarray analysis [Battaglia et al., 2008; Maas et al., 2008; South et al., 2008c; Zollino et al., 2008]. Clinical severity generally correlates with deletion sizes, although co-existing duplicated chromosomal regions, sequence variation of the gene(s) in the non-deleted 4p region, and other genetic backgrounds can all contribute to phenotypic variation [South et al., 2008c; Zollino et al., 2008]. Seizures represent a major clinical challenge in patients with WHS [Battaglia et al., 2009]. A review by Zollino et al. [2008] showed a high prevalence of seizures in patients with WHS regardless of the deletion sizes: 96% in those with <3.5 Mb deletions, 80% in those with 5–18 Mb deletions, and 90% in those with >22 Mb deletions. However, it remains unclear whether the severity of seizures in WHS patients might correlate with deletion sizes.
A WHS critical region, responsible for cardinal WHS features such as distinctive faces, growth/developmental delay, and seizures, was initially mapped to a 165-kb interval (WHSCR1) involving the entire WHSC2 gene and the proximal part of WHSC1 [Wright et al., 1997]. Additional reports of WHS patients with deleted regions distal to WHSCR1 suggested a new critical region (WHSCR2) involving the distal part of WHSC1 and the entire LETM1 gene, and these two genes have been considered the molecular hallmark of WHS [Zollino et al., 2003; Rodriguez et al., 2005]. Indeed, WHSC1 is hypothesized as the gene that contributes to the main WHS phenotype of developmental delay and characteristic facial features [Nimura et al., 2009; Izumi et al., 2010]. LETM1, encoding a mitochondrial Ca2+/H+ antiporter [Jiang et al., 2009], is thought to be the major candidate gene for seizures in patients with WHS [Rauch et al., 2001; South et al., 2007], while FGFRL1, located distal to WHSCR1/WHSCR2 and involved in bone cartilage formation during embryonic development, might be another candidate gene for craniofacial features of WHS [Catela et al., 2009; Engbers et al., 2009]. Other genes localized around these critical regions might also contribute to the various features of WHS.
Here, we present detailed clinical, microarray, and FISH-based molecular-cytogenetic findings of 22 Japanese patients with WHS, representing the first large series in a non-Western country.
MATERIALS AND METHODS
Patients
Twenty-two WHS patients from eight hospitals were included in this study between January 2010 and February 2012. The diagnosis of WHS was made from clinical characteristics as well as G-banded karyotyping with or without FISH analysis using a WHSCR or 4p subtelomeric probe. Written informed consent was obtained from all parents of the patients. Clinical information was collected by the clinical geneticists of each hospital and reviewed by one of them (K.S.). Ethical approval for this study was granted by the Institutional Review Board of Shinshu University School of Medicine, Matsumoto, Japan.
Chromosomal Microarray Analysis
Genomic DNA was isolated using standard protocols from the peripheral blood leukocytes of each patient. Chromosomal microarray analysis was performed through two whole genome oligonucleotide-based array platforms. NimbleGen CGX Array™ (Roche NimbleGen, Inc., Madison, WI) was used in the analyses of 21 patients, and includes 134,829 probes with an average resolution of 35 kb throughout the genome and 10 kb in clinically significant regions. Procedures for DNA labeling and microarray analysis were performed according to the manufacturer's instructions. The fluorescence signals on array slides were analyzed using a NimbleScan™ (Roche NimbleGen, Inc.), and presentation of array results was obtained by Genoglyphix® Software (Signature Genomics Laboratories, Spokane, WA). The Agilent Human Genome Microarray Kit 244A™ (Agilent Technologies, Santa Clara, CA) was used in the analysis of one patient, and includes 243,504 probes with a median probe space of 7.4 and 13.4 kb in intragenic and intergenic genomic sequences, respectively. Labeling and hybridization were performed according to the manufacturer's instructions, followed by scanning with an Agilent Microarray Scanner™ and data extraction with Feature Extraction Software™ (v9.5.3). The results were analyzed using Cytogenomics 2.0 Software™ (Agilent Technologies). Genomic coordinates of both array results were indicated according to NCBI build 36 (hg 18).
Metaphase FISH Analysis
To confirm cytogenetic rearrangements resulting in 4p deletion, FISH analysis using Bacterial Artificial Chromosome (BAC) probes was performed on metaphase chromosomes from peripheral blood leukocytes of all patients. We selected BAC probes around deleted regions of 4p and around other terminal duplicated segments in patients who were predicted to have unbalanced rearrangements according to microarray results. Parental blood samples, where available, were also assayed with metaphase FISH to define whether chromosomal derivatives were de novo or inherited from parental balanced rearrangements. When a mosaic chromosomal abnormality was detected by G-banded karyotyping or suspected by chromosomal microarray results that showed a lower absolute log2 ratio than observed in patients with complete deletions or duplications, FISH analysis was performed on over 30 cells to detect a mosaic ratio.
RESULTS
Molecular-Cytogenetic Findings
Molecular-cytogenetic findings are summarized in Table I. G-banded chromosome analysis at the 400–550 band level, which was performed prior to this study, was abnormal in 18/22 (82%) patients: with terminal deletions of 4p in eight patients, interstitial deletions in three, additional materials of unknown origin attached to 4p in six, and mosaic chromosomes for a del(4)(p15.3p16.1) cell line (interstitial deletion) and a normal 46,XX cell line in Patient 15. G-banded chromosomes were normal in other patients who were found to have a deletion of 4p by FISH analysis using a 4p subtelomeric probe.
| Patient no. | G-Banded chromosomes | Array-CGH analysis | Final 4p rearrangement studied with a-CGH and FISH | Inheritance | ||||
| Location and minimal deletion size of 4p | Distal breakpoint from 4ptera | Proximal breakpoint from 4pter | Other unbalanced region and minimal size | |||||
| 1 | 46,XX | 4p16.3 | 2.06 Mb | 1–(63,075) | 2,061,942–2,071,068 | dup(10)(q26.3), 772 kb | Unbalanced translocation der(4)t(4;10)(p16.3,q26.3) | |
| 2 | 46,XX | 4p16.3 | 2.29 Mb | 1–(41,413) | 2,294,435–2,313,104 | Isolated terminal | ||
| 3 | add(4)(p16) | 4p16.3 | 2.93 Mb | 1–(63,075) | 2,934,119–2,941,688 | dup(4)(q31.22q35.2), 45.6 Mb | der(4)(4qter→q31.22:p16.3→qter) | |
| 4 | 46,XX | 4p16.3p16.2 | 3.45 Mb | 1–(63,075) | 3,453,423–3,475,088 | Isolated terminal | ||
| 5 | 46,XX | 4p16.3p16.1 | 5.26 Mb | 1–(63,075) | 5,259,705–5,286,063 | Isolated terminal | ||
| 6 | del(4)(p16.1) | 4p16.3p16.1 | 5.49 Mb | 1–(33,860) | 5,488,869–5,517,610 | Isolated terminal | De novo | |
| 7 | del(4)(p16.1) | 4p16.3p16.1 | 6.92 Mb | 1–(33,860) | 6,920,095–6,944,615 | Isolated terminal | ||
| 8 | del(4)(p16.1) | 4p16.3p16.1 | 7.51 Mb | 1–(63,075) | 7,509,074–7,525,657 | Isolated terminal | ||
| 9 | del(4)(p15.3p16) | 4p16.3p16.1 | 8.09 Mb | 1–(63,075) | 8,089,462–8,126,238 | Isolated terminal | ||
| 10 | add(4)(p15.3) | 4p16.3p16.1 | 8.77 Mb | 1–(63,075) | 8,772,114–9,414,321 | Isolated terminal | ||
| 11 | add(4)(p15.2) | 4p16.3p16.1 | 8.77 Mb | 1–(33,860) | 8,772,114–9,414,321 | dup(8)(p23.3p23.1), 6.9 Mb | Unbalanced translocation der(4)t(4:8)(p16.1;p23.1) | Maternal |
| 12 | del(4)(p16.1) | 4p16.3p16.1 | 8.77 Mb | 1–(33,860) | 8,772,114–9,414,321 | Isolated terminal | De novo | |
| 13 | add(4)(p15.3) | 4p16.3p16.1 | 8.85 Mb | 1,329,023–1,370,178 | 10,219,850–10,254,956 | Isolated interstitial | ||
| 14 | add(4)(p15.2) | 4p16.3p15.33 | 11.11 Mb | 1–(63,075) | 11,105,238–11,129,635 | Isolated terminal | De novo | |
| 15 | 46,XY,del(4)(p15.3p16.1) [26]/46,XY[4] | 4p16.3p15.33 | 12.01 Mb | 1–(33,860) | 12,006,591–12,044,138 | mos.del(4)(p15.33)/del(4)(p16.3) | ||
| 16 | del(4)(p15.3) | 4p16.3p15.33 | 12.33 Mb | 1–(33,860) | 12,331,994–12,371,059 | Isolated terminal | De novo | |
| 17 | del(4)(p15.32p16.3) | 4p16.3p15.33 | 13.63 Mb | 813,367–841,095 | 14,467,735–14,498,501 | Isolated interstitial | De novo | |
| 18 | add(4)(p15.2) | 4p16.3p15.32 | 15.70 Mb | 1–(63,075) | 15,700,625–15,737,006 | Isolated terminal | ||
| 19 | del(4)(p15.3) | 4p16.3p15.31 | 18.62 Mb | 1–(63,075) | 18,616,970–18,655,860 | Isolated terminal | De novo | |
| 20 | del(4)(p15.2p16) | 4p16.3p15.31 | 21.00 Mb | 1–(63,075) | 20,992,651–21,031,043 | dup(11)(q25), 1.27 Mb | mos.der(4)t(4;11)(p15.31;q25)/del(4)(p15.31) | De novo |
| 21 | del(4)(p15.1) | 4p16.3p15.1 | 28.35 Mb | 1–(33,860) | 28,348,051–28,384,613 | Isolated terminal | De novo | |
| 22 | del(4)(p15.1) | 4p16.3p15.1 | 29.42 Mb | 1–(63,075) | 29,416,450–29,451,155 | Isolated terminal | ||
- Genomic locations of array results are according to NCBI build 36 (hg 18).
- a Distal breakpoints of 41,413, 33,860, and 63,075 actually show terminal 4p deletions because the probes designed for the region are nonspecific to 4pter.
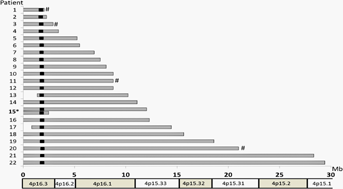
Chromosomal microarray analysis revealed 4p deletions to be terminal in 20 patients and interstitial in the other 2 patients. Deletion sizes ranged from 2.06 to 29.42 Mb, and all included WHSCR2 (Fig. 1). In patients with 4p terminal deletions, those ≤5.26 Mb were not detected by G-banded karyotyping. Only three patients (Patients 10, 11, and 12) shared the same breakpoints between 8.77 Mb (minimum) and 9.41 Mb (maximum) from the 4p terminus, corresponding to the loci of olfactory receptor (OR) gene clusters. Duplicated chromosomal regions accompanied by 4p deletion included 772 kb of terminal 10q in Patient 1, 45.6 Mb of terminal 4q in Patient 3, 6.9 Mb of terminal 8p in Patient 11, and 1.27 Mb of terminal 11q in Patient 20. In Patient 20, log2 values for probes spanning the duplicated 11q region were approximately 0.365 (theoretical log2 value of non-mosaic duplication, 0.58), which suggested that the duplication was mosaic (Fig. 2A).
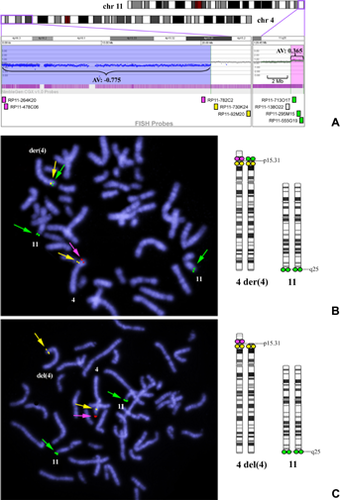
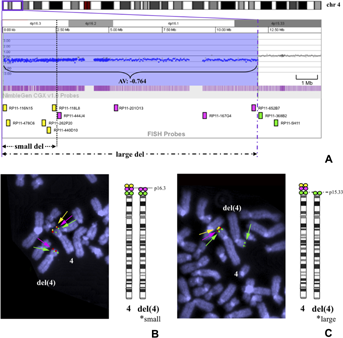
FISH analysis using BAC probes designed according to microarray results confirmed a derivative chromosome 4 consisting of a duplicated 4q segment on the deleted 4p in Patient 3, and unbalanced translocations between 4p and 10q in Patient 1, and between 4p and 8p in Patient 11. In Patient 20, metaphases with an unbalanced translocation, der(4)t(4;11)(p15.31;q25), were found in 22/30 cells; and those with a simple terminal deletion, del(4)(p15.31), were found in 8/30 cells. The breakpoint of 4p was considered to be identical in both cell lines (21.0 Mb from 4pter; Fig. 2B, C). In Patient 15, metaphases with del(4)(p15.33) (11.9–12.1 Mb deletion) were found in 45/56 cells and those with del(4)(p16.3) (2.48–2.66 Mb deletion) were found in 11/56 cells (Fig. 3B, C), although only the larger deletion was demonstrated in microarray analysis (Fig. 3A). In nine patients whose parental samples were available, eight were found to have de novo deletions and the other (Patient 11) was found to have a maternal unbalanced translocation.
Clinical Findings and Correlation With Genotype
Clinical findings are summarized in Table II and major structural defects are listed according to deletion size in Table III. We categorized patients according to their deletion sizes into “small” with 4p terminal deletions less than 6 Mb (Patients 1–6), “intermediate” with deletions ranging from 6 to 15 Mb (Patients 7–17), and “large” with deletions over 15 Mb (Patients 18–22).
| Patient no. | Age/sex | Minimal deletion sizes in 4p(Mb) | Accompanied duplicated regions | Seizure onset | Status epilepticus | Treatment at the time of this study/course of seizures | CNS complications | Developmental delay |
|---|---|---|---|---|---|---|---|---|
| 1 | 6y/F | 2.06 | dup(10q),772 kb | +/9m | – | CZP/disappeared | – | Moderate (DQ41)/walk at 2y3m |
| 2 | 13y/F | 2.29 | +/2y6m | – | VPA/disappeared for 5 years | N.I. | Severe (IQ23)/walk at 7y | |
| 3 | 1y8m/F | 2.93 | dup(4q),45.6 Mb | +/1y3m | – | –/disappeared after only one attack | – | Severe/no sitting |
| 4 | 4y3m/F | 3.45 | +/7m | + | VPA, CLB/occurred frequently | Periventricular leukomalacia | Severe/no head control | |
| 5 | 5y10m/F | 5.26 | +/1y1m | – | CZP/well-controlled | N.I. | Moderate/walk at 4y | |
| 6 | 16y/F | 5.49 | +/2y1m | – | DZP/disappeared since 8y | N.I. | Severe/walk at 7y | |
| 7 | 18y/F | 6.92 | +/6m | + | VPA, Br, Vit B6 (West syndrome)/improved after Br at 1y | – | Severe (IQ10)/walk at 7y | |
| 8 | 6y6m/M | 7.51 | +/7m | + | TPM, PB, CLB, Br/improved after Br at 3y | N.I. | Severe/roll over at 1y5m | |
| 9 | 5y3m/F | 8.09 | +/10m | + | PB, VPA/occurred frequently | – | Severe/head control at 12m | |
| 10 | 7m/M | 8.77 | – | – | −/− | – | Severe | |
| 11 | 8y/F | 8.77 | dup(8p),6.9 Mb | +/6m | + | VPA, PB/improved after 2y | – | Severe (DQ10)/sit at 4y |
| 12 | 16y/F | 8.77 | +/7m | + | VPA, PHT/improved after 2y | Ventricular enlargement | Severe/head control at 2y | |
| 13 | 12y/F | 8.85 (1.37–10.22) | – | −/− | – | Moderate/walk at 2y3m | ||
| 14 | 5y/F | 11.11 | +/9m | + | VPA, Br/well-controlled | – | Severe/walk | |
| 15 | 9m/M | 12.02 | +/8m | + | VPA/continued | Ventricular enlargement | Severe | |
| 16 | 1y11m/F | 12.33 | +/9m | + | VPA, LGT, CZP/occurred frequently | HCC, Cerebellar atrophy | Severe (DQ30)/head control at 9m | |
| 17 | 18y/F | 13.63 (0.84–14.5) | +/1y | + | −/disappeared for several years | HCC | Severe (IQ10)/walk at 5y | |
| 18 | 3y/F | 15.70 | +/1y2m | + | VPA, CLB, Br/improved gradually | – | Severe/head control | |
| 19 | 2y11m/F | 18.60 | +/2m | + | PB, CZP, TPM/occurred frequently | – | Severe/no head control | |
| 20 | 4y/F | 21.00 | dup(11q),1.27 Mb | +/1m | – | VPA, ZNS/disappeared for two years | Cerebral atrophy | Severe/no head control |
| 21 | 2y10m/F | 28.35 | +/11m | + | TPM, CLB/disappeared since 2y | Cerebral atrophy | Severe | |
| 22 | 6y/M | 29.42 | +/2m | + | VPA, ESM, PB/occurred frequently | HCC, Grey matter heterotopia, white matter volume loss | Severe (DQ10)/no head control |
| Patient no. | Height/weight (SD)a | Feeding | CL/CP | Heart | Urogenital | Skeletal | Ophthalmologic | Hearing impairment | Other complication(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −2.4/−2.0 | Oral | − | ASD | − | − | Strabismus | − | |
| 2 | −5.4/−3.5 | Oral | − | ASD | N.I. | Scoliosis (mild) | − | − | Multiple osteochondromatosis |
| 3 | −5.3/−2.3 | Tube | − | − | − | − | − | Severe | hypercholesterolemia |
| 4 | −4.3/−3.0 | Tube | − | ASD, PDA, VSD | N.I. | Limited hip flexion | Strabismus | Moderate | |
| 5 | −3.8/−2.9 | Oral | − | ASD | N.I. | − | Strabismus | − | Hypercholesterolemia |
| 6 | −4.9/−3.0 | Oral | − | − | − | N.I. | Strabismus (ET) | − | |
| 7 | −5.1/−2.5 | Oral | − | PS | Renal hypoplasia, RF | Pes planus | − | Moderate | Hypercholesterolemia, hyperuricemia |
| 8 | −6.4/−3.7 | Tube | − | − | − | − | NDO | Moderate | Fanconi syndrome due to VPA |
| 9 | −5.8/−4.1 | Oral | − | ASD, PS | − | − | Strabismus | Moderate | |
| 10 | −4.5/−3.9 | Tube | + (CP) | PDA | − | − | Right cataract | − | |
| 11 | −4.1/−3.3 | Tube | + (SMCP) | AR | − | N.I. | Strabismus, NDO | − | |
| 12 | −11.7/−5.3 | Tube | + (CL/CP) | PS | Renal hypoplasia, RF | Scoliosis (mild) | Strabismus (XT) | Severe | |
| 13 | −3.0/−2.3 | Tube | − | VSD | − | − | − | ||
| 14 | −5.2/−4.0 | Oral | ASD | − | − | − | Moderate | ||
| 15 | −2.0/−2.3 | Oral | − | ASD, PS | Criptorchidism | – | N.I. | Moderate | |
| 16 | –3.4/–2.7 | Tube | + (SMCP) | ASD, PDA | Hydronephrosis | Talipes varus | Strabismus (XT), NDO | – | |
| 17 | –5.3/–3.8 | Oral | – | ASD | Renal hypoplasia, RF | Acetabular dysplasia | Cataract | – | Hypercholesterolemia |
| 18 | –5.6/–4.0 | Tube | – | ASD, PS | Renal hypoplasia, RF | Sagittal craniosynostosis | Coloboma | – | Hypercholesterolemia |
| 19 | –4.4/–2.4 | Tube | – | ASD, PS | – | Scoliosis | Optic nerve atrophy | Moderate | |
| 20 | –3.3/–2.6 | Tube | – | PDA, VSD | Renal hypoplasia VUR | – | – | Moderate | |
| 21 | –2.3/–3.1 | GS | +(CP) | ASD, PDA | UJS, RF | Talipes varus Cervical spine abnormalities | – | Severe | |
| 22 | –1.3/–1.4 | GS | + | ASD | Renal dysplasia, RF, cryptorchidism, hypospadias | – | Cataract, coloboma | Severe |
- ASD, atrial septal defect; Br, potassium/sodium bromide; CL, cleft lip; CLB, clobazam; CNS, central nervous system; CP, cleft palate; CZP, clonazepam; DQ, developmental quotient; DZP, diazepam; ESM, ethosuximide; ET, esotropia; F, Female; GS, gastrostomy; HCC, hypoplasia of the corpus callosum; IQ, intelligence quotient; LGT, lamotrigine, M, Male; m, months; NDO, nasolacrimal duct obstruction; N.I., not investigated; PB, phenobarbital; PDA, patent ductus arteriosus; PHT, phenytoin; PS, pulmonary stenosis; RF, renal failure; SMCP, submucous cleft palate; TPM, topiramate; UJS, ureteropelvic junction stenosis; VPA, valproate; VSD, ventricular septal defect; VUR, vesicoureteric reflux; XT, exotropia; y, years; ZNS, zonisamide.
- a Height and weight were evaluated at the time of this study.
| Small (<6 Mb) | Intermediate (6–15 Mb) | Large (>15 Mb) | |
|---|---|---|---|
| Congenital heart defects | 4/6 (67%) | 10/11 (91%) | 5/5 (100%) |
| Renal abnormalities [renal failure] | 0/3 (0%) [0/3] | 4/11 (36%) [3/11] | 4/5 (80%) [3/5] |
| Ocular defects | 0/6 (0%) | 2/11 (18%) | 3/5 (60%) |
| Cleft lip/palate | 0/6 (0%) | 5/11 (45%) | 2/5 (40%) |
| Skeletal anomalies | 2/6 (33%) | 4/11 (36%) | 3/5 (60%) |
Neurological Features
Seizures began in 20/21 (95%) patients within the first three years of life (1 month to 2 years and 6 months old). Patient 10 was excluded because he was only 7 months old at the time of this study and might be expected to develop seizures in the future. Only Patient 13, aged 12 years with an 8.8 Mb interstitial deletion, had no seizures. The onset of seizures was late, occurring over 1 year of age, in 4/6 (67%) patients with small deletions (<6 Mb); and it was early, under 1 year of age, in 12/14 (86%) patients with larger deletions (>6 Mb). Status epilepticus occurred in 14/20 (70%) patients: 1/6 (17%) in those with small deletions and 13/14 (93%) in those with larger deletions. Seizures were intractable in six patients of ages ranging from 9 months to 6 years (median, 3 years and 7 months old), while seizures improved or disappeared in 14 patients of ages ranging from 1 year and 8 months to 18 years (median, 6 years and 2 months old). Potassium or sodium bromide was administered to four patients and the daily dose of bromide was 450 mg in Patient 7, 560 mg in Patient 8, 200 mg in patient 14, and 400 mg in Patient 18 at the time of this study. Seizures decreased in all of these. Patient 7 and Patient 8 showed a particularly obvious improvement after bromide therapy started at the ages of 1 year and 3 years, respectively. Neuroimaging demonstrated structural central nervous system defects in 9/18 (50%) patients, including periventricular leukomalacia, ventricular enlargement, hypoplasia of the corpus callosum, and cerebral or cerebellar atrophy.
A total of 19/22 (86%) patients showed severe developmental delay, while the other 3 patients showed a moderate delay. Independent walking was achieved in seven patients, including one with no seizures and five with no history of status epileptics.
Other Clinical Findings
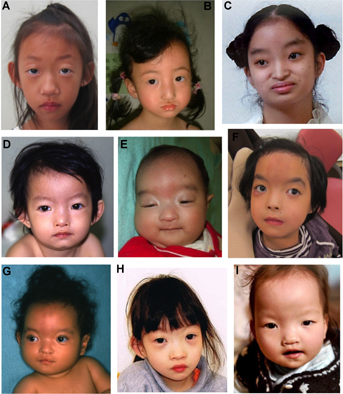
Typical craniofacial features were present in all the patients except for Patient 1 with the smallest sized deletion (2.06 Mb; Fig. 4A) and Patient 13 with an interstitial deletion (1.37–10.22 Mb; Fig. 4H), both showing subtle features without the Greek warrior helmet appearance. The severity of short stature varied among patients regardless of the deletion sizes and the deleted regions. Eleven patients with various deletion sizes required tube feeding because of insufficient oral feeding associated with hypotonia, poorly coordinated swallowing, and/or gastroesophageal reflux. Gastrostomy was performed in two patients with the largest and the second largest deletions because of persistent insufficient oral feeding, failure to thrive regardless of tube feeding, recurrent respiratory distress, hypoglycemia, and/or carnitine deficiency.
Congenital heart defects were seen in 19/22 patients (86%). Three patients without heart defects had smaller deletions of 2.93, 5.49, and 7.51 Mb. The observed heart defects were common types, including atrial septal defects in 13 patients, pulmonary stenosis in six, and patent ductus arteriosus in five. The severity of the heart defects was not correlated with deletion sizes or deleted regions. No life-threatening complex heart defects were present in this series.
Structural urogenital anomalies were detected in a total of 47% (9/19) patients, including renal hypoplasia or dysplasia in six. The prevalence of patients with renal hypoplasia or dysplasia was 0/3 (0%) in the small deletion-type, 3/11 (27%) in the intermediate, and 3/5 (60%) in the large. Renal hypoplasia or dysplasia resulted in renal failure in five patients (83%).
Ophthalmologic abnormalities were detected in a total of 62% patients (13/21). Strabismus and nasolacrimal obstruction were found in patients with small or intermediate deletion-types, whereas structural ocular anomalies were found in patients with intermediate (2/11, 18%) and large (3/5, 60%) deletion-types. The prevalence of cleft lip/palate was 32% (7/22), that of skeletal abnormalities was 45% (9/20), and that of hearing impairment was 55% (12/22).
Other complications included hypercholesterolemia (>220 mg/dl) in a total of 36% (5/14) patients in whom serum cholesterol levels were examined, and the hypercholesterolemia was not familial in all the patients except one, whose parental information was not available. Multiple osteochondromatosis was observed in Patient 2 with a terminal 2.29 Mb deletion. The age of onset of osteochondromatosis was around 2 years. Radiological examination revealed a cartilage-capped bony growth arising from the area of the growth palate of the distal tibia and from the surface of the scapula. The patient underwent several surgical resections for progressive osteochondromatosis. Direct sequencing and multiplex ligation-dependent probe amplification analysis of EXT1 and EXT2, the genes responsible for multiple osteochondromatosis (multiple exostosis type I (OMIM#133700) and type II (OMIM#133701), respectively) [Francannet et al., 2001], showed no pathogenic sequence variants.
DISCUSSION
In the current study, we performed microarray and FISH-based molecular-cytogenetic investigations of 22 Japanese patients with WHS, coupled with a detailed and comprehensive clinical evaluation. This resulted in identification of previously unreported complex chromosomal mosaicism and implication of several findings about the genotype–phenotype correlation including severity of seizures and structural anomalies.
Unbalanced translocations combined with a 4p deletion or other complicated rearrangement were identified in a total of four (18%) patients, which was lower than the recently reported frequency of 45% (15/33) by South et al. [2008c]. This discrepancy might be attributable to selection bias in both studies or the possible presence of a translocation with acrocentric p-arm in the current study, which could be detected through silver staining of the nucleolar organizing region (NOR), FISH using alpha satellite DNA probes, or parental FISH studies using a WHS-specific 4p16.3 probe [South et al., 2008c].
Hitherto unreported patterns of complex mosaicism for two different structurally abnormal cell lines were identified in two of the patients in our current series using a combination of G-banding, microarray, and metaphase FISH analysis. Only a limited number of mosaicism cases have been previously reported of two cell lines both carrying 46 chromosomes and different structurally abnormal chromosomes, including del(8p)/inv dup del(8p) in several patients [Vermeesch et al., 2003; Pramparo et al., 2004; Hand et al., 2010]. Only one WHS patient was reported to carry two different structurally abnormal cell lines, del(4)(p16)/der(4)(qter-q31.3::pter–qter), which might have resulted from a meiotic crossing over event causing the der(4) cell line to be associated with a pericentric inversion and subsequent mitotic breakage [Syrrou et al., 2001]. A previous report showed expansion of a 4p terminal deletion between a mother and a son [Faravelli et al., 2007] and a subsequent report described this on 18q [South et al., 2008b]. Patient 15 might be another example of apparent instability of a terminal deletion, representing the expansion of a deletion within an individual rather than between generations.
Mosaicism of two different structurally abnormal cell lines, del(4)(p15.31)/der(4)t(4;11)(p15.31;q25), was indicated in Patient 20 by microarray through the lower log2 ratio of the duplicated 11q region. This supports the utility of microarray in that it can detect not only small copy number variation at a significantly higher resolution, but also detect mosaicism by incomplete log2 ratio compared with complete deletion or duplication [Ballif et al., 2006]. By contrast, mosaicism could not be detected in Patient 15 by microarray, perhaps because of the different levels of mosaicism between the two patients: 22:8 in Patient 20 and 45:11 in Patient 15.
Our study included four patients with other duplicated chromosomal regions detected by microarray. The duplicated segment of 10q26.3–qter (772 kb) in Patient 1, 11q25–qter (1.27 Mb, mosaicism) in Patient 20, and 8p23.1–pter (6.9 Mb) in Patient 11 have not been reported to associate with extensive disease pathology in a trisomic state [Engelen et al., 2000; Harada et al., 2002; Iwanowski et al., 2011]. The 45.6 Mb duplication at the 4q31.22–qter region in Patient 3 is considered to be mainly associated with psychomotor delay and often with cardiac and renal anomalies [Otsuka et al., 2005; Wang et al., 2009]. Indeed, Patient 3 showed severe developmental delay in spite of a small 4p deletion (2.93 Mb), but no apparent cardiac or renal anomaly.
The severity of seizures is evaluated from the time of onset and the presence of status epilepticus. Six patients with small deletions (<6 Mb) from 4pter tended to have a later onset of seizures and status epilepticus was less common than those patients with intermediate (6–15 Mb) or large deletions (>15 Mb). Developmental delay was severe in most patients, with the exception of three with a moderate delay: two of these had small terminal deletions (2.06 and 5.26 Mb) and one had an intermediate interstitial deletion (8.85 Mb). Seizure severity is, therefore, suggested to correlate with the 4p deletion size, which might result in correlation between severity of developmental delay and the 4p deletion size.
Candidate region(s) for seizures in patients with WHS and possible responsible genes are shown in Figure 5. Although LETM1 is presently considered to be the major responsible gene for seizures [Endele et al., 1999; Rauch et al., 2001; South et al., 2007], the more distal region of the chromosome has also been suggested as a candidate region for seizure penetrance [South et al., 2008a; Misceo et al., 2012]. Indeed, Patient 13 in our series did not have seizures and had an interstitial deletion (1.37–10.22 Mb from 4pter) encompassing LETM1 but preserving the distal regions, which is similar to “Case 6” reported by Maas et al. [2008] with an interstitial deletion (1.3–2.5 Mb) including LETM1 and no seizures. Four patients with seizures were reported to have small distal 4p deletions not including LETM1 [Faravelli et al., 2007; Maas et al., 2008; Zollino et al., 2008; Misceo et al., 2012]. Considering a patient with ring chromosome 4 and a 4p terminal deletion of 760 kb not experiencing seizures [Concolino et al., 2007], the susceptible gene(s) for seizures in WHS might be localized in the region between 760 kb and 1.3 Mb from the 4pter. In our series, Patient 17 with an interstitial deletion encompassing both LETM1 and most of the new candidate region had severe seizures.
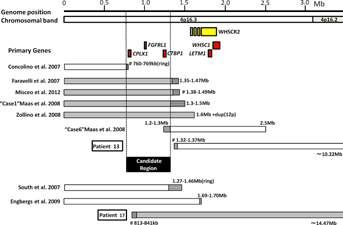
CTBP1 and CPLX1 are localized in this new susceptible region for seizures. CTBP1 encodes a transcriptional corepressor that acts at the promoters of many genes [Chinnadurai, 2007]. In an epileptogenic rat model, a ketogenic diet as well as 2-deoxy-D-glucose, a glycolysis-inhibiting drug, reduces epilepsy by stimulating Ctbp activity. Ctbp co-operates with transcriptional factor NRSF to repress expression of BDNF, a strongly suspected epileptogenic signaling molecule [Garriga-Canut et al., 2006]. The hemizygosity of CTBP1 in WHS patients is therefore considered a potential contributor to the progression of epilepsy [Simon and Bergemann, 2008]. CPLX1 encodes a type of complexin that binds to syntaxin within the SNARE complex and regulates the fusion of synaptic vesicles [McMahon et al., 1995]. Homozygous Cplx1 deletion mutant mice develop strong ataxia and sporadic seizures [Reim et al., 2001; Glynn et al., 2005]. These findings suggest that CTBP1 and CPLX1 as well as LETM1 could be susceptibility genes for seizures in WHS.
Bromide therapy was previously reported to be an effective antiepileptic drug in four patients with WHS, in whom it was shown to reduce status epilepticus [Kagitani-Shimono et al., 2005]. In the current study, four patients were administered bromide therapy, which was effective in all. In particular, Patients 7 and 8 showed a marked reduction in seizure frequency after the initiation of bromide therapy. Further information including the types or severity of seizures, electroencephalography (EEG) patterns, efficacy of treatment, and microarray-based deletion mapping in a larger patient series will be necessary to establish a detailed seizure phenotype–genotype correlation.
Hypercholesterolemia, which has not been reported in previous studies, was observed in five patients in the present study, suggesting it to be a noteworthy complication of WHS. LRPAP1, localized 3.5 Mb from 4pter, was deleted in four of the patients. LRPAP1 encodes LDL receptor-related protein-associated protein 1 that plays an important role in lipoprotein metabolism [Willnow et al., 1995], and might therefore be related to hypercholesterolemia. Multifactorial inheritance, including nutritional problems, could also be related to the occurrence of hypercholesterolemia.
In conclusion, this genotype–phenotype correlation study using microarray and FISH-based molecular-cytogenetic investigations uncovered chromosomal rearrangements in all patients including previously unreported complex chromosomal mosaicism. It also demonstrated the correlation of deletion size from 4pter with seizure severity and with occurrence of renal hypoplasia/dysplasia and structural ocular anomalies, and described additional clinical features including hypercholesterolemia. Moreover, a new susceptible region distal to the previously-supposed candidate gene LETM1 was suggested for the occurrence of seizures, and the usefulness of bromide therapy was stressed for seizure management. To prevent intractable seizures and status epileptics, patients with 4p deletion involving the new susceptible region as well as LETM1 are recommended to have careful EEG follow-up and intensive pharmacological treatment based on the seizure occurrence and EEG findings, including application of bromide therapy. These findings are relevant to the improvement of WHS healthcare guidelines, as well as to the elucidation of gene(s) function in the deleted region.
ACKNOWLEDGMENTS
We thank the patients and their families for participating in this study. This work was supported by Research on Intractable Diseases from Japanese Ministry of Health, Labour and Welfare (K.T., Y.F.).




