Correlation of intercentromeric distance, mosaicism, and sexual phenotype: Molecular localization of breakpoints in isodicentric Y chromosomes†
How to Cite this Article: Beaulieu Bergeron M, Brochu P, Lemyre E, Lemieux N. 2011. Correlation of intercentromeric distance, mosaicism, and sexual phenotype: Molecular localization of breakpoints in isodicentric Y chromosomes. Am J Med Genet Part A 155: 2705–2712.
Abstract
Isodicentric chromosomes are among the structural abnormalities of the Y chromosome that are commonly identified in patients. The simultaneous 45,X cell line that is generated in cell division due to instability of the isodicentric Y chromosome [idic(Y)] has long been hypothesized to explain the variable sexual development of these patients, although gonads have been studied in only a subset of cases. We report here on the molecular localization of breakpoints in ten patients with an idic(Y). Breakpoints were mapped by FISH using BACs; gonads and fibroblasts were also analyzed when possible to evaluate the level of mosaicism. First, we demonstrate great tissue variability in the distribution of idic(Y). Second, palindromes and direct repeats were near the breakpoint of several idic(Y), suggesting that these sequences play a role in the formation of idic(Y). Finally, our data suggest that intercentromeric distance has a negative influence on the stability of idic(Y), as a greater proportion of cells with breakage or loss of the idic(Y) were found in idic(Y) with a greater intercentromeric distance. Females had a significantly greater intercentromeric distance on their idic(Y) than did males. In conclusion, our study indicates that the Y chromosome contains sequences that are more prone to formation of isodicentric chromosomes. We also demonstrate that patients with an intercentromeric distance greater than 20 Mb on their idic(Y) are at increased risk of having a female sexual phenotype. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Isodicentric Y chromosomes [idic(Y)] are one of the most common structural abnormalities of the Y chromosome [Hsu, 1994]. Highly unstable during mitosis due to the presence of two centromeres, most idic(Y) chromosomes are found in mosaics with a 45,X cell line [Hsu, 1994; Tuck-Muller et al., 1995; Bouayed Abdelmoula and Amouri, 2005a]. It has been hypothesized that the proportion of 45,X cells in various tissues would likely influence the phenotypic sex of individuals bearing an idic(Y) chromosome, which ranges from infertile men with hypospadias, to Turner syndrome in women, to individuals with ambiguous genitalia [Hsu, 1994; Tuck-Muller et al., 1995; Bouayed Abdelmoula and Amouri, 2005b].
Isodicentrics caused by a break in the short arm, thus having two intact copies of the long arm of the Y, are abbreviated idic(Y)(p), while isodicentrics caused by a break in the long arm, thus having two intact copies of the short arm, are abbreviated idic(Y)(q) according to the International System for human Cytogenetic Nomenclature (ISCN) [2009]. However, when referring to an idic with unspecified breakpoints, some authors use idicY(p) or idic(Yp) when two copies of the short arm are present, and idicY(q) or idic(Yq) when two copies of the long arm are present, therefore creating some confusion among readers. In the present paper, all abbreviations are written following the ISCN nomenclature that is idic(Y)(p) and idic(Y)(q).
As part of a study on structural abnormalities of the Y chromosome, we mapped by fluorescence in situ hybridization (FISH) the isodicentric Y chromosome breakpoints in ten patients (six males and four females). This allowed us to correlate the phenotypic sex of patients with the intercentromeric distance of idic(Y), as well as the instability of the rearranged Y chromosomes.
CLINICAL REPORTS
The study comprised ten patients, four females and six males. All four females were found to have pure gonadal dysgenesis and underwent prophylactic bilateral removal of gonads and Fallopian tubes. The males had variable features. Patient 1 presented with cryptorchidism; Patient 4 had mixed gonadal dysgenesis, with his left gonad containing both dysgenetic testicular tissue and ovarian tissue. The remaining four male patients had a normal sexual phenotype. Patients 3 and 10 were previously published by DesGroseilliers et al. [2006] as patients D1 and D8, respectively.
MATERIALS AND METHODS
The study was approved by the Ethics Committee of the Centre Hospitalier Universitaire (CHU) Sainte-Justine, a pediatric hospital in Montreal. Peripheral blood lymphocytes, amniocytes, skin fibroblasts, and cells from fresh gonadal tissues were prepared according to standard protocols [modified from Rooney and Szepulkowski, 1992]. Paraffin-embedded gonadal tissues were prepared using proteinase K and 30% sodium bisulfite, [modified from DesGroseilliers et al., 2006]. The GTG banding was done according to Lemieux et al. [1990] and karyotypes were established following guidelines of ISCN [2009]. For FISH, the following commercial probes were hybridized as suggested by manufacturers: Yq12 heterochromatin [DYZ1 (Oncor, Gaithersberg, MD) or CEP Y Satellite III (Vysis, Downers Grove, IL)], Y centromere [CEP Y Alpha Satellite (Vysis) or DYZ3 (Oncor)], Y subtelomeric regions [TelVysion Xp/Yp (DXYS129) and Xq/Yq (ESTCdy16c07) (Vysis)], the SRY-region [SRY (Vysis)], X centromere [CEP X (Vysis) or DXZ1 (Oncor)], centromeric regions of chromosome 18, X and Y [AneuVysion Probe Mixture #1 (Vysis)], and the pan-telomeric probe recognizing the consensus sequence (TTAGGG)n of human telomeres [FITC-(C3TA2)3 peptide nucleic acid (DAKO, Mississauga, ON, Canada)]. We also hybridized a 4.5 kb Hind III fragment of the DYZ4/DYZ5 repeat locus in Yp11.2 [Tyler-Smith et al., 1988], cosmid clones LLycos130G04 and cos37C09 containing sequences specific to the pseudoautosomal region II (PAR2) of the X and Y chromosomes [Kühl et al., 2001] and the following BAC clones from the Human library RPCI-11 according to the UCSC Genome Browser 2006 hg18 assembly (http://genome.ucsc.edu): RP11-145K2, RP11-702H14, RP11-117L2, RP11-1077B13, RP11-148G1, RP11-652A7, RP11-80C13, RP11-91A13, RP11-1122B19, RP11-78A5, RP11-360H3, RP11-80M6, and RP11-462A19 (Yq11.221); RP11-20H21, RP11-601J1, RP11-138F16, RP11-914P19, RP11-483G19, RP11-356K22, RP11-5I7, and RP11-256K9 (Yq11.222); RP11-120E18, RP11-95B23, RP11-5C5, RP11-140H23, RP11-120I15, RP11-141N4, and RP11-761P22 (Yq11.223); RP11-214M24 (Yq11.223-11.23); RP11-1077O23 and RP11-945P24 (Yq11.23). All hybridizations were done simultaneously on a control slide [modified from Lemieux et al., 1992]. The distance between the centromeres of each idic(Y) was estimated by multiplying by two the distance separating the breakpoint and the centromere, based on the localization of the breakpoint in the Genome Browser. In cases where the breakpoint was mapped in Yq12, the breakpoint was estimated to be in the middle of heterochromatin, as done by Lange et al. [2009]. Pearson's and Spearman's correlations were performed using the SPSS 16.0 software for MacIntosh (SPSS Inc., Chicago, IL).
RESULTS
Final karyotypes obtained on blood or amniocytes and intercentromeric distances of idic(Y) are presented in Table I, while FISH results are shown in Figure 1. Breakpoints for each patient are represented in Figure 2 and the proportions of the various cell lines are illustrated in Figure 3.
| Patient | Sex | Karyotype | Intercentromeric distance |
|---|---|---|---|
| 1 | M | 46,X,del(Y)(q11)[28]/46,X,idic(Y)(p11.32)[30]/45,X[11] | 22 Mb |
| 2 | M | 46,X,idic(Y)(p11.32)[183]/45,X[47] | 22 Mb |
| 3 | M | 46,X,idic(Y)(q11.221)[140] | 7 Mb |
| 4 | M | 47,XX,idic(Y)(q11.221)[108]/46,XX[274] | 12 Mb |
| 5 | M | 46,X,idic(Y)(q11.222)[193]/45,X[6]/47,X,idic(Y)(q11.222)x2[1] | 17 Mb |
| 6 | F | 46,X,idic(Y)(q11.223)[183] | 24 Mb |
| 7 | F | 46,X,idic(Y)(q11.23)[72]/45,X[160]/46,X,del(Y)(q11)[1] | 31 Mb |
| 8a | M | 46,X,idic(Y)(q11.23)[41]/45,X[22] | 31 Mb |
| 9 | F | 46,X,idic(Y)(q12)[138]/45,X[36]/46,X,del(Y)(q12)[1] | 60 Mb |
| 10 | F | 46,X,idic(Y)(q12)[260]/45,X[21]/47,X,idic(Y)(q12)x2[3] | 60 Mb |
- a Only amniocytes were available for this patient.
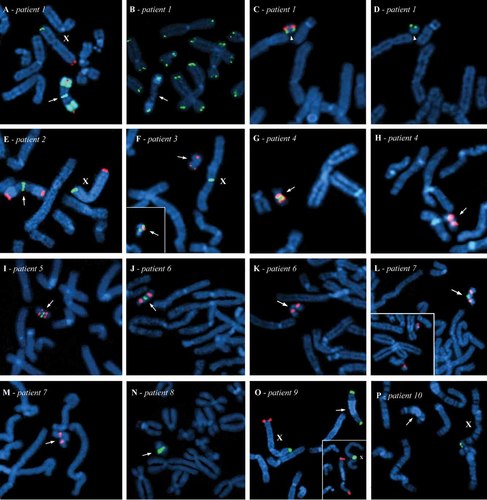
Partial metaphases showing FISH with commercial probes, BACs, and/or cosmid clones. Arrows point to the breakpoint on the idic(Y) while arrowheads point to the mar(Y); the X chromosome is identified when visible. Patient 1: (A) Presence of Yq subtelomeres (red) and Yq12 heterochromatin (green) in their normal position on the idic(Y), as well as Yp subtelomeres (green) at the breakpoint; (B) absence of pan-telomeres (green) at the breakpoint on the idic(Y). Presence of (C) SRY-region (red), Y centromere (green) (D), and Yp subtelomeres (green) on the mar(Y). Patient 2: (E) Presence of Yq subtelomeres (red) in their normal position on the idic(Y) and of Yp subtelomeres (green) and at the breakpoint. Patient 3: (F) BAC RP11-117L2 (Yq11.221; green) is absent on the idic(Y) marked by probe SRY (red); the X centromere is in green. Subset: BAC RP11-702H14 (Yq11.221; red) is present on the idic(Y) marked by probe DYZ4/DYZ5 (green). Patient 4: (G) Presence of BAC RP11-1122B19 (Yq11.221; green) and (H) absence of BAC RP11-78A5 (Yq11.221; green) on the idic(Y) marked by the Y centromere (red). Patient 5: (I) BAC RP11-601J1 (Yq11.222; green) is present on the idic(Y) identified by the Y centromere (red). Patient 6: (J) Presence of BAC RP11-5C5 (Yq11.223; green) and (K) absence of BAC RP11-140H23 (Yq11.223; green) on the idic(Y) marked by the Y centromere (red). Patient 7: (L) BAC RP11-1077O23 (Yq11.23; green) is present on the idic(Y) identified by the Y centromere (red). Subset: Breakage of the idic(Y) hybridized with the Y centromere (red); the intercentromeric fragment hybridized with BAC RP11-1077O23 (Yq11.23; green) was lost. M: Absence of BAC RP11-945P24 (Yq11.23; green) on the idic(Y) marked with a Y-centromeric probe (red). Patient 8: (N) Presence of BAC RP11-1077O23 (Yq11.23; green) on the idic(Y). Patient 9: (O) Absence of Yq subtelomeres (red) at the breakpoint on the idic(Y); Yp subtelomeres (green) are found in their normal position. Subset: Breakage of the idic(Y) in Yq12. The idic(Y) is hybridized with SRY (red) whereas the X centromere is in green. Patient 10: (P) Cosmid clone for PAR2 (green) is present at the distal end of the X chromosome, but is absent on the idic(Y).
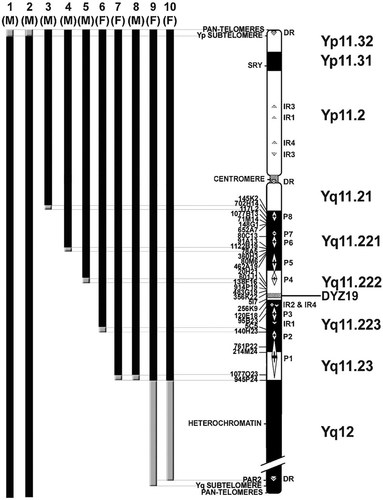
Ideogram of the Y chromosome with localization of FISH probes on the left, as well as position of palindromes (P) 1–8, inverted repeats (IR) 1–4, and direct repeats (DR) on the right. Black bars indicate chromosome segments present in each patient whereas gray bars indicate breakpoint regions. The sexual phenotype, male (M) or female (F), is indicated for each patient. Although idic(Y) consist, by definition, of two mirror-copies of the same sequence, only one copy is depicted for simplicity.
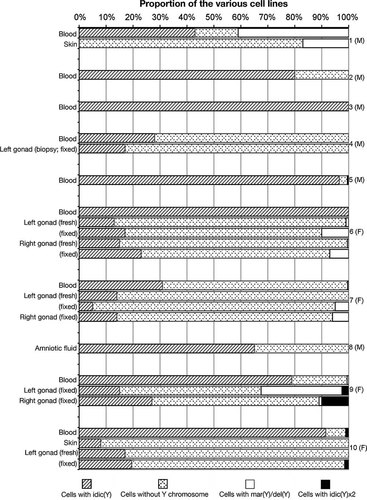
Bar chart representing the proportion of various cell lines in blood lymphocytes, amniocytes, skin fibroblasts, fixed and/or fresh left and/or right gonadal tissue of all ten patients. Tissues are specified on the left; patients are indicated on the right with their sexual phenotype.
FISH with a probe for the SRY-region showed symmetric signals in all ten cases: Interstitial on the idic of Patients 1 and 2 [idic(Y)(p)] and at both ends of the idic in Patients 3–10 [idic(Y)(q)]. As for FISH with the Y centromere, it confirmed the dicentric nature of the rearranged Y in all ten patients.
The FISH analysis on the idic(Y) of Patient 1 indicated that Yp subtelomeres were present at the breakpoint (Fig. 1A), whereas pan-telomeres were not present (Fig. 1B). The breakpoint was thus localized between the subtelomeres and pan-telomeres, at 0–0,264 Mb in Yp11.32. This idic(Y) chromosome was found in 43% of his blood lymphocytes; a marker chromosome (mar) was also found in 41% of the blood cells, while the remaining cells were 45,X. This mar was positive for probes specific to the Y centromere, SRY-region (Fig. 1C) and Yp subtelomeres (Fig. 1D), but negative for Yq subtelomeres and pan-telomeres (not shown). As for the patient's fibroblasts, 17% showed only the mar(Y), whereas all the remaining cells were 45,X. Unfortunately, we were not able to study the gonads of this patient as all hybridization attempts on the testis biopsy failed.
The idic(Y) of Patient 2 was found to be very similar to the one found in Patient 1. Indeed, Yp subtelomeres were present at the breakpoint (Fig. 1E) but not the pan-telomeres (not shown). The breakpoint was thus also in Yp11.32, between the subtelomeres and the pan-telomeres, at 0–0,264 Mb. Eighty percent of blood lymphocytes carried the idic(Y); the remaining cells were 45,X. No marker chromosome was found.
Blood lymphocytes from Patient 3 were found to have a homogeneous idic(Y). The breakpoint was localized at the beginning of Yq11.221, between BACs RP11-702H14 (Fig. 1F subset) and RP11-117L2 (Fig. 1F), at 14,637–14,853 Mb. As for Patient 4, a 47,XX,idic(Y) constitution was found in 28% of his lymphocytes. However, loss of the idic(Y) was associated with a 46,XX cell line that was found in a majority of cells. As FISH was positive with BAC RP11-1122B19 (Fig. 1G) on the idic(Y) but was negative with BAC RP11-78A5 (Fig. 1H), the breakpoint for this patient is at 17,144–17,305 Mb, in the distal half of Yq11.221. The 46,XX cell line was also found to be predominant in FISH analyses performed on the fixed biopsy of his left dysgenetic testis. Patient 5 had an idic(Y) in 97% of his blood cells. This idic(Y) was positive for BAC RP11-601J1 (Fig. 1I). However, no hybridization signal was observed with BAC RP11-138F16 (not shown). We concluded that the breakpoint was at 19,915–20,060 Mb, in the middle of Yq11.222.
Patient 6 was found to have an homogeneous idic(Y) in her blood cells. BAC RP11-5C5 (Fig. 1J) hybridized on the idic(Y), whereas BAC RP11-140H23 (Fig. 1K) did not. Therefore, breakage occurred in the middle of Yq11.223, at 23,387–23,612 Mb. The idic(Y) was also found in 14–21% of her gonadal cells, whereas a subset of cells (0.5–2%) contained only one signal for the Y centromere, possibly indicating the presence of a del(Y). The remaining gonadal cells had lost the idic(Y) and were 45,X. As these del(Y) cells were found both on fresh and fixed tissue from both sides, this tends to confirm that this cell line was present, albeit at a low proportion.
A FISH experiment with probes specific to the X and Y centromeres showed the presence of an idic(Y) in 31% of the blood lymphocytes from Patient 7, as well as in 65% of the amniocytes from Patient 8. The remaining cells were 45,X in both patients. BAC RP11-1077O23 (Fig. 1L and N) was present on both idic(Y), but BAC RP11-945P24 was absent in both Patients 7 (Fig. 1M) and 8 (not shown). The breakpoint was thus at 26,856–27,001 Mb, near the end of Yq11.23 in both patients. Furthermore, in the case of Patient 7, one metaphase of blood lymphocytes showed a clear break of the idic(Y), resulting in two del(Y) (Fig. 1L subset). The idic(Y) was found in 12–14% of cells in both gonads, and a possible del(Y) was also found in approximately 2% of her gonadal cells. Unfortunately, no tissues other than amniotic fluid were available from Patient 8.
In Patients 9 and 10, heterochromatin Yq12 was clearly present on the idic(Y) found in 79% and 93%, respectively, of their blood cells. The FISH with Yq subtelomeres was negative in both Patients 9 (Fig. 1O) and 10 (not shown). Hybridization with cosmid clones for the PAR2 region was also negative on the idic(Y) of Patient 10 (Fig. 1P), but could not be performed in Patient 9 due to lack of material. The breakpoint was thus localized within Yq12 heterochromatin, proximal to the subtelomeres in both patients. An idic(Y) was also found in the patients' fibroblasts (Patient 10) and gonadal tissue (Patients 9 and 10), although in a minority of cells. In both patients, the 45,X cell line was predominant in the gonads. Furthermore, 30% of nuclei from the paraffin-embedded left gonad and 1% from the right gonad of Patient 9 were found to contain only one signal for the Y centromere, suggesting the presence of a del(Y). However, hybridization proved to be difficult on the left gonad and only a few cells could be analyzed. Clear visualization of one cell showing a break in the Yq12 region of the idic(Y) of Patient 9 (Fig. 1O subset) in blood lymphocytes, as well as another cell showing fragility in the same region, confirms the presence of a del(Y) cell line in this patient. Finally, 10% of cells from the right gonad of Patient 9 were found to have four signals for the Y centromere, suggesting the presence of two idic(Y) in these cells; this was not seen in blood. As for Patient 10, two signals for the Yq12 heterochromatin, compatible with the presence of two idic(Y), were seen in a subset of gonadal cells. This was also found in a similar proportion in blood lymphocytes.
A correlation of the proportion of cells that lost the Y chromosome in various tissues for each patient to the intercentromeric distance revealed that female patients had a significantly greater intercentromeric distance than did males (Pearson's correlation r = 0.615; P = 0.001). Also, a Spearman's rank correlation of the intercentromeric distance and 45,X mosaicism in blood or fixed gonads, excluding Patient 4, yielded a P < 0.1 and a r of 0.472.
DISCUSSION
Although idic(Y) are one of the most common structure abnormalities of the Y chromosome, breakpoints have been assigned to a specific cytogenetic band in only a third of published cases, more than half of theses being published recently [Lange et al., 2009]. In fact, Lange et al. [2009] not only characterized the molecular breakpoints of idic(Y), they found that 56 of the 78 idic(Y) they investigated had breakpoints in palindromes, which are susceptible to recombination. Similarly, in our study, palindromes and/or inverted repeats are found at or very near the breakpoint of four out of ten of the idic(Y) cases (Patients 3, 6, 7, and 8; Fig. 2). However, palindromes are not implicated in the formation of all idic(Y), as indicated both by our results and those of Lange et al. [2009]. Regions of chromatin more prone to breakage are known to exist, such as common fragile sites that are characterized by the presence of AT-rich sequences [reviewed in Lukusa and Fryns, 2008]. As extremities of Yq12 heterochromatin are thought to be composed of DYZ2 repeats [Ludeña et al., 1993], which are made of an AT-rich sequence interspersed with Alu repeats [Cooke et al., 1982; Frommer et al., 1984], the Y chromosome may be more susceptible to breakage in that region. In fact, two of our patients (Patients 9 and 10) have breakpoints in Yq12, as well as two other patients also reported by Lange et al. [2009]. At least nine other cases of idic(Y) with breakpoints in Yq12 have been published [reviewed in Hsu, 1994; Jakubowski et al., 2000; DesGroseilliers et al., 2002; Hsieh et al., 2002]. Furthermore, several direct repeats are found on the Y chromosome, whether at the distal end of both the short and long arms or at the centromere (Fig. 2). The breakpoints in Patients 1 and 2 reported here were found in the terminal region of Yp, which contains a direct repeat (Fig. 2), but we could not determine the exact location of the breakpoint within a 264 kb interval due to lack of specific cytogenetic probes. It is possible that these repeats are prone to breakage and may go through a simple U-type exchange. Indeed, Codina-Pascual et al. [2004] also reported a patient where the breakpoint was between the subtelomeres and telomeres. In addition, Lange et al. [2009] reported a few cases of Y isochromosomes that seem to have breakpoints in or around the direct repeats found at the Y centromere. For the two remaining patients (Patients 4 and 5) of our cohort, no repeats were found in the breakpoint region of Patient 5 (Fig. 2) that could have been more susceptible to breakage. As for Patient 4, the last BAC present on the idic(Y) of this patient overlapped with the distal border of palindrome 6 (Fig. 2), indicating that the breakpoint was very close to a palindrome; whether or not this played a role in the formation of the chromosome rearrangement remains unclear. Therefore, although regions such as the Yq12 heterochromatin, palindromes and direct repeats might confer susceptibility to the formation of idic(Y), these sequences were not implicated in all cases. This was also found by Lange et al. [2009], as 20 of the 78 idic(Y) chromosomes they studied had breakpoints outside of such sequences.
Although idic(Y) have been shown to be unstable during mitosis [Cohen et al., 1973; Buchanan et al., 1976], further rearrangements of these chromosomes due to breakage–fusion–bridge (BFB) cycles are not frequently seen. In fact, presence of a marker, ring, deleted, or isochromosome Y aside from the idic(Y) has rarely been reported [Tuck-Muller et al., 1995; Bouayed Abdelmoula and Amouri, 2005a]. In this paper, we report that four of ten patients (Patients 1, 6, 7, and 9) presented a further rearrangement of their idic(Y) in their blood, skin, and/or gonads. These results indicate that idic(Y) are indeed unstable and can be further rearranged, although rarely in a great proportion of cells, making them difficult to detect. Furthermore, the proportion of these cell lines can vary greatly among tissues and was generally higher in gonads than in blood, which might explain why few cases have been reported since gonads are not frequently analyzed. On the contrary, complete loss of the idic(Y) is more frequent, especially in females, in all types of tissues (Figs. 3 and 4).
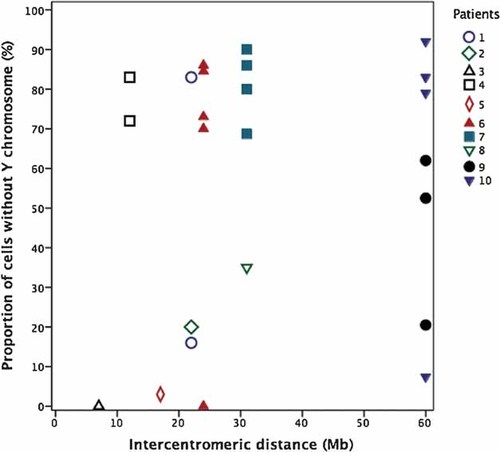
Correlation of the proportion of cells that lost the Y chromosome in various tissues for each patient to the intercentromeric distance. Each patient is represented by a specific symbol: Males by open symbols and females by filled ones.
As we knew the location of the breakpoint from the FISH analyses, we were able to estimate the intercentromeric distance for all patients (Table I). Although our study size is modest, the results suggest that the intercentromeric distance is correlated with the stability of idic(Y). The female patients of the cohort had a significantly greater intercentromeric distance than did the males (Pearson's correlation r = 0.615; P = 0.001), all female patients having an intercentromeric distance of more than 20 Mb (Fig. 4). This is in agreement with the observations of Lange et al. [2009]. Greater intercentromeric distance could predispose both to loss and breakage of the idic(Y). Indeed, centromeres of the same chromatid may bind to spindles from opposite mitotic poles, generating abnormal tension of the idic(Y), as originally proposed by Ying and Ives [1971]. The greater the distance between centromeres, the greater are the chances of reorientation of the idic(Y) on the metaphase plate [Buchanan et al., 1976]. This could result in breakage [del(Y) or mar(Y) cell line] or anaphase lag, with subsequent loss of the idic(Y) in a micronucleus [45,X cell line] or migration of both copies in the same daughter cell [45,X/47,X,idic(Y)x2 cell lines]. As pointed out by Lange et al. [2009], stabilization of idic(Y) through centromere inactivation would therefore be of greater importance as intercentromeric distances increase, since only active centromeres bind to mitotic spindles. In our study, all patients with a mar(Y) or del(Y) cell line (Patients 1, 6, 7, and 9) had an intercentromeric distance of more than 20 Mb on their idic(Y) (Table I).
Approximately 50% of patients with an idic(Y) develop as females, whereas 20% of patients have ambiguous genitalia. Thus, gonadal 45,X mosaicism was hypothesized to be responsible for the disorder of sex development of many patients bearing an idic(Y) (reviewed in [Hsu, 1994; Tuck-Muller et al., 1995; Bouayed Abdelmoula and Amouri, 2005b]). However, gonads have been studied in only a few cases, as reviewed by Bouayed Abdelmoula and Amouri [2005b and DesGroseilliers et al. [2006], and tissue mosaicism was unfortunately not studied by Lange et al. [2009]. In our cohort of ten idic(Y) patients, all four females had 45,X mosaicism in 50% or more of their gonadal cells, both fresh or fixed, as well as an intercentromeric distance of more than 20 Mb (Figs. 3 and 4). As for Patient 4, who had a 47,XX,idic(Y)/46,XX karyotype in his blood with a predominant (72%) 46,XX cell line, FISH analyses performed on a biopsy of his left gonad showed that the 46,XX cell line was predominant at 83% (Fig. 3). A later gonadectomy showed the presence of both ovarian and dysgenetic testicular tissue. Although this patient had a dysgenetic testis with ovarian tissue, he nonetheless had a male phenotype. The absence of a more feminized phenotype in spite of the predominance of a 46,XX cell line is puzzling; this suggests that other factors played a role in the sexual development of this patient. When performing a Spearman's rank correlation of the intercentromeric distance and 45,X mosaicism in blood or fixed gonads, the two most common tissues in our cohort, a statistical trend (r = 0.472; P < 0.1) was observed after exclusion of Patient 4 due to his atypical sexual phenotype. Further studies on larger cohort are necessary to confirm our data.
In conclusion, our results indicate that idic(Y) breakpoints often occur near palindromes or regions containing repeats, suggesting that these sequences play a role in the formation, or at least confer susceptibility to these structural abnormalities. Our data also suggest that intercentromeric distance has a negative influence on the stability of idic(Y), as a greater proportion of cells with breakage or loss of the idic(Y) were found in idic(Y) with an intercentromeric distance of more than 20 Mb. The female patients described here were found to have a significantly greater intercentromeric distance than did the men, as well as increased loss of the idic(Y), especially in their gonads. Therefore, this suggests that patients with an intercentromeric distance greater than 20 Mb on their idic(Y) are at increased risk of having a female phenotype due to extensive 45,X gonadal mosaicism.
Acknowledgements
The authors thank Fléchère Fortin and Claude Potvin for their help and support, as well as Dr. Bruno Maranda, of the Centre Hospitalier Universitaire de Québec (CHUQ), for his collaboration. They are grateful to Dr. Christopher Tyler-Smith for providing them with probe DYZ4/DYZ5, and to Drs. Werner Schempp and Rainer Wimmer for cosmid clones LLycos130G04 and cos37C09. MBB received grants from Le Fonds de Recherche en Santé du Québec (FRSQ), La Fondation du CHU Sainte-Justine and La Fondation des Étoiles. Purchase of BACs was financed in part by Le Réseau de Médecine Génétique Appliquée (RMGA).




