Chromosomal anomalies in the etiology of anorectal malformations: A review†
How to Cite this Article: Marcelis C, de Blaauw I, Brunner H. 2011. Chromosomal anomalies in the etiology of anorectal malformations: A review. Am J Med Genet Part A 155: 2692–2704.
Abstract
Anorectal malformation (ARM) is a severe congenital anomaly that can occur either isolated or in association with other congenital abnormalities. It has a heterogeneous etiology with contribution of both genetic and environmental factors, although the etiological factors remain largely unknown. Several chromosomal abnormalities have been described in patients with an ARM. These chromosomal abnormalities could point to specific genes involved in the development of the anorectal canal and associated structures. This paper reviews the chromosomal abnormalities described in ARM and may act as a starting point to identify chromosomal regions containing putative anorectal development genes. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Anorectal malformation (ARM) is a relatively frequent abnormality of hindgut development with an estimated incidence of 1 in 2500 live births [Spouge and Baird, 1986; Cuschieri, 2001]. The incidence of associated anomalies varies between 45% and 65% [Spouge and Baird, 1986; Hassink et al., 1996; Endo et al., 1999; Cuschieri, 2002]. Most commonly associated anomalies involve the genitourinary tract, central nervous system, skeleton (vertebrae/limbs), cardiovascular system and the remaining gastrointestinal tract [Spouge and Baird, 1986; Cuschieri, 2002].
ARM is a multifactorial disorder for which the individual environmental or genetic risk factors are still largely unknown. A number of monogenetic syndromes have an ARM as one of the possible features and already point to the involvement of specific genes in hindgut development. Martuciello provides a good review of some well know syndromes with an ARM [Martuciello, 2006]. Chromosome abnormalities are present in 4.5–11% of the patients with ARM [Spouge and Baird, 1986; Cho et al., 2001; Cuschieri, 2002]. In this article we will review our experience with chromosomal abnormalities in a series of patients with an ARM and combine that with a literature review and review of cases of ARM within the European Cytogeneticists Association Register of Unbalanced Chromosome Aberrations (ECARUCA) database for chromosomal abnormalities.
Radboud University Nijmegen Medical Centre Hospital-Database
The clinical characteristics of all patients born with an ARM and treated in our hospital have been collected since 1974. A previous study [Hassink et al., 1996] described the data from the cases gathered between 1974 and 1995. These 264 patients included nine patients with a chromosomal abnormality (3.4%), 5 of these had a trisomy 21. Since 1995 an additional 254 ARM cases have been added to the database, including 17 with a chromosomal abnormality (6.7%). Overall this means 26 chromosomal abnormalities were identified among a total of 518 ARM cases (5.0%). Table I reviews the chromosomal abnormalities in these series. As expected the most frequent chromosomal abnormality was trisomy 21 in 11 cases. Two cases had a velocardiofacial syndrome with a microdeletion 22q11.2. The only other recurrent chromosomal abnormality in our cohort is 5p-deletion syndrome (Cri-du-chat), which was present in three cases. One of these had an unbalanced translocation with a monosomy for the proximal part of chromosome 14 and a deletion 5p15. Cuschieri [2002] mentions an additional three cases of 5p deletion in his review on ARM with additional anomalies but no other cases have been published and a literature review on Cri-du-chat syndrome did not reveal additional cases with an ARM.
| Chromosome abnormality | Male | Female | Total |
|---|---|---|---|
| Trisomy 21 | 5 | 6 | 11 |
| Trisomy 18 | — | 1 | 1 |
| Del 1pter | — | 1 | 1 |
| Del 1q23–25 | — | 1 | 1 |
| Del 2q22 | — | 1 | 1 |
| Del 5p15.1 | — | 2 | 2 |
| 45,XX,der(5)t(5;14)(p15;q13), -14 | — | 1 | 1 |
| Trisomy 5p | — | 1 | 1 |
| Del 6q25.3 | — | 1 | 1 |
| Del 10q26 | — | 1 | 1 |
| Partial trisomy 11q | — | 1 | 1 |
| Del 13q33.2–q34 | 1 | — | 1 |
| Del 22q11.2 (VCF) | — | 2 | 2 |
| Idic22q11.2 (Cat-Eye) | 1 | — | 1 |
| Unknown marker | — | 1 | 1 |
| Total | 7 | 18 | 25 |
ECARUCA Database
The ECARUCA database is an online database for rare chromosomal abnormalities [Feenstra et al., 2006] (http://www.ecaruca.net). It contains cytogenetic and clinical data of patients with rare chromosomal abnormalities, both microscopically visible as well as microdeletions and -duplications. It currently has information on 4,600 cases and 2,650 unique chromosome aberrations. It does not contain data on the most common autosomal trisomies (13, 18, and 21) and X and Y chromosome abnormalities, unless combined with another autosomal aberration. Many of the cases have also been described in the “Catalogue of Unbalanced Chromosome Aberrations in Man” [Schinzel, 2001] and most of these have previously been published elsewhere. This database has the opportunity to search on clinical features. A search on anal atresia revealed 121 cases that have an ARM as one of their features. Of these, 86 had an isolated abnormality and 35 had a complex rearrangement. For cases that were previously published, we reviewed the original articles. 1
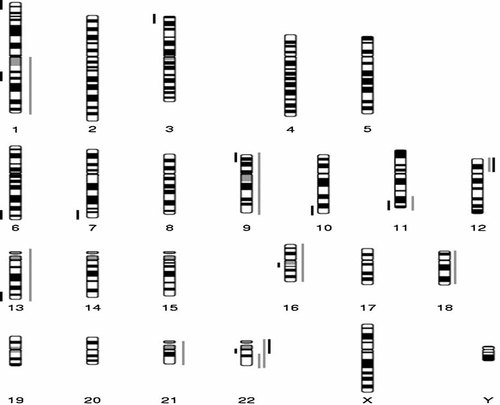
Chromosomal imbalances in multiple patients with anorectal malformations. Deleted regions are depicted (in black) to the left of the chromosome. Duplicated (light gray) or triplicated (black) are depicted to the right of the chromosome.
Literature Search
The PubMed electronic database of the US National Library of Medicine was searched using different combinations of anal atresia, anorectal (malformation) or imperforate anus with chromosome, followed by the numbers 1–22. The terms copy number variation (CNV), gain, loss, microdeletion, and microduplication were also combined with the different terms for ARM to decrease the chance of missing smaller deletions and duplications. References of the articles found in this way were reviewed to identify studies that might have been missed. When multiple cases of a similar chromosomal anomaly with ARM were found, literature on that chromosomal anomaly and the ECARUCA database were reviewed to estimate the frequency of ARM in patients with that anomaly. Only chromosomal anomalies present in 2 or more cases will be discussed in detail. Figure one gives a schematic representation of these recurrent chromosomal anomalies. We only included cases with anal atresia/imperforate anus in our original search and did not include anal stenosis or anteriorly displaced anus (ADA). The main reason for this is that anal atresia/imperforate anus is easily recognized by physicians, while anal stenosis and especially anterior displaced anus can easily be missed or misinterpreted. However we did look for the occurrence of additional cases with these (minor) anomalies when we reviewed the literature on a specific chromosomal anomaly and we comment on that in the text when appropriate. A list of all identified published reports of patients with anal atresia/imperforate anus and a chromosomal anomaly is shown in Table II.
| Chr. | Loss | Gain | Refs. |
|---|---|---|---|
| 1 | dup1q21-qter | Scheuerle et al. 2005 | |
| dup1q21-qter | Wax et al. 2000 | ||
| del1q24q25.3 | Takano et al. 1997 | ||
| del2q31-qter | dup1p31-pter | Halal et al. 1989 | |
| 2 | del2q12q14 | Antich et al. 1983 | |
| del2q37-qter | Stratton et al. 1994 | ||
| 3 | del3q12.2q13.2 | Kosaki et al. 2005 | |
| del3p25.3-pter | Asai et al. 1992 | ||
| del3p25-pter | Reifen et al. 1986 | ||
| 4 | dup4pter4qter | Van Allen et al. 1993 | |
| 5 | del5q31.3qter | dup10q26qter | Lazjuk et al. 1985 |
| 6 | del6q25.3-qter | Titomanlio et al. 2006 | |
| del6q23-q25 | McLeod et al. 1990 | ||
| del6q26/del6p25 | Peeden et al. 1983 | ||
| del6q27/del6p26 | Dawson et al. 1995 | ||
| del6q24.3-qter | Meng et al. 1992 | ||
| Del6q26-qter | Dup7q31.2-qter | Choy et al. 2009 | |
| dup6q21qter | Stamberg et al. 1981 | ||
| 7 | del7p15.2-p21 | Kosaki et al. 2005 | |
| del20q13.3-qter | dup7p15-pter | Reish et al. 1996 | |
| 9 | del9p24pter | Muroya et al. 2000 | |
| del9p23-pter | Fujimoto et al. 2004 | ||
| del9q34.1qter | Thauvin-Robinet et al. 2004 | ||
| dup9pterqter | Delicado et al. 1985 | ||
| 10 | del10q25.3-qter | Wulfsberg et al. 1989 | |
| del10q26.3-qter | Wulfsberg et al. 1989 | ||
| del10q25.2qter | Wegner et al. 1981 | ||
| del10q26.3-qter | dup9q32-qter | Tsukuda et al. 1996 | |
| del10q26.3-qter | dup11q14.2-qter | Maruyama et al. 2001 | |
| dup10q11.21–22.3 | Lam et al. 2000 | ||
| 11 | del11q23-qter | Puvabanditsin et al. 2001 | |
| del11q24.1-qter | von Bubnoff et al. 2004 | ||
| del11q24-qter | Leegte et al. 1999 | ||
| Del11q25-qter | Dup16q13-qter | Hatanaka et al. 1984 | |
| dup11q23-qter | Smeets et al. 1997 | ||
| 12 | trip12p | Reynolds et al. 1987 | |
| trip12p | Wenger et al. 1990 | ||
| trip12p | Baglaj et al. 2008 | ||
| trip12p | McLeod et al. 1991 | ||
| trip12p | Pauli et al. 1987 | ||
| trip12p | Tejada et al. 1992 | ||
| dup12p12.1-q12 | Cinti et al. 2001 | ||
| dup12p11-pter | Stengel-Rutkowski et al. 1981 | ||
| 13 | del13q? | Allderdice et al. 1969 | |
| del13q32.2qter | Bartsch et al. 1996, Garcia et al. 2006 | ||
| del13q32q34 | Bartsch et al. 1996 | ||
| del13q13qter | Benn et al. 1983 | ||
| del13q? | Biles et al. 1970 | ||
| del13q22qter | dup15q26qter | Brown et al. 1993 | |
| del13q22q34 | Brown et al. 1993 | ||
| del13q32qter | Christofolini et al. 2006 | ||
| del13q33.2qter | Garcia et al. 2006 | ||
| del13q33qter | Garcia et al. 2006 | ||
| del13q22qter | Gershoni-Baruch and Zekaria 1996 | ||
| del13q31.3qter | Kirchhoff et al. 2009 | ||
| del13q31.2qter | Kirchhoff et al. 2009 | ||
| del13q33.2-qter | Kuhnle et al. 2000 | ||
| del13q22-qter | Niikawa et al. 1980 | ||
| del13q13.3qter | Quelin et al. 2009 | ||
| del13q21.32qter | Quelin et al. 2009 | ||
| del13q31.1q33.3 | Quelin et al. 2009 | ||
| del13q31.1qter | Quelin et al. 2009 | ||
| del13q31.1qter | Walsh et al. 2001 | ||
| 16 | dup16pterqter (2) | Vaughan et al. 1994 | |
| dup16pterqter | Yancey et al. 1996 | ||
| dup16pterqter | Seller et al. 2004 | ||
| dup16pterqter | Kalousek et al. 1993 | ||
| dup16pterqter | Cusick et al. 1995 | ||
| dup16pterqter | Eggermann et al. 2004 | ||
| dup16q11-qter | Ridler and McKeown 1979 | ||
| del18p11.2-pter | dup16q21-qter | Garau et al. 1980 | |
| del16q11.2q12.2 | Bardakjian et al. 2009 | ||
| del16q24.1 | Stankiewicz et al. 2009 | ||
| 18 | del18p11.2-pter | dup20q13.3qter | Su et al. 2006 |
| gain18q10-q11.2 | van der Veeken et al. 2010 | ||
| dup18q10qter | Chen et al. 1998 | ||
| dup18p11.21-18q12.1 | Schramm et al. 2011 | ||
| 20 | dup13q12.3/dup20p13 | Nagai et al. 1994 | |
| 21 | del21pter-q21 | Abe et al. 1990 | |
| 22 | del22q11.2 | Yamagishi et al. 1998 | |
| del22q11.2 | Worthington et al. 1997 | ||
| del22q11.2 | Schulze et al. 2001 | ||
| dup11/dup22 | Prieto et al. 2007 | ||
| dup11/dup22 | Schinzel et al. 1981b | ||
| dup11/dup22 (2) | Fraccaro et al. 1980 | ||
| dup11/dup22 (2) | Iselius et al. 1983 | ||
| dup11/dup22 (2) | Zackai and Emanuel 1980 | ||
| trip22pter-q11 (5) | Liehr et al. 1992 | ||
| trip22pter-q11 | Hoo et al. 1986 | ||
| trip22pter-q11 (2) | Ing et al. 1987 | ||
| trip22pter-q11 (9) | Schinzel et al. 1981a | ||
| trip22pter-q11 | Crolla et al. 1997 | ||
| trip22pter-q11 | Verschraegen-Spae et al. 1993 | ||
| trip22pter-q11/ DupYq | Gabarron et al. 1985 | ||
| dup22pterqter | Iselius and Faxelius 1978 | ||
| dup22pterqter | Schinzel 1981 | ||
| dup22pterqter | Slater et al. 1993 |
- A number in parentheses following chromosomal gain or loss indicates the number of patients with this chromosomal abnormality reported by the associated reference when there was more than one affected patient.
Chromosome 1
Deletion 1p36-pter
Our series has one case with a terminal 1p deletion, the most common terminal deletion syndrome, detected using MLPA studies. She has multiple congenital anomalies with, besides an ARM, microcephaly, tricuspid valve atresia with a large ventricular septum defect, hydronephrosis, bicornuate uterus with hydrometocolpos and low lumbar and sacral spinal defects. At age 1 year her psychomotor development was much better than expected in a 1p deletion patient. A review of the literature revealed one case with anal stenosis [Slavotinek et al., 1999] and intestinal obstruction. Recently an additional patient with OEIS complex associated with a 1p36 deletion was described [El-Hattab et al., 2010]. In a review of 60 patients with a 1p36 deletion three patients with anal anomalies are described; two with anteriorly displaced anus, one with imperforate anus [Battaglia et al., 2008]. In another review no anal anomalies are described in 59 cases, but constipation is a frequent feature [Gajecka et al., 2007].
Deletion 1q24–25
One of our patients was a girl with a de novo deletion 1q23–25 and anal atresia without fistula. A case with an overlapping interstitial deletion (del(1)(q24q25.3)) and an ARM was described in the literature [Takano et al., 1997] and present in the ECARUCA database.
Duplication 1q
Two cases of almost complete trisomy 1q presenting with an ARM have been described in the medical literature. Scheuerle et al. described a case with an imperforate anus without fistula in which mosaicism for an unbalanced Y;1 translocation was found prenatally. Postnatal karyotype was normal, but additional FISH studies were consistent with the mosaic prenatal findings for trisomy 1q [Scheuerle et al., 2005]. Wax et al. [2000] described a case of trisomy 1q with imperforate anus, ambiguous genitals and sacrococcygeal teratoma with mosaicism for an unbalanced (1;15) translocation.
Chromosome 3
Deletion 3p25
Only two cases of a terminal deletion of chromosome 3p (del3p25) and an ARM are published [Reifen et al., 1986; Asai et al., 1992]. Multiple cases of the 3p25 deletion syndrome have been published and no additional cases with an ARM have been reported.
Chromosome 6
Deletion 6q25.3
Several patients with a terminal 6q deletion and an ARM are reported in the literature. The patient presented by Choy et al. [2009] in addition had a partial trisomy 7q. The patients that were presented by McLeod et al. [1990] and Titomanlio et al. [2006] in addition had sacral agenesis. Titomanlio et al. report a possible ARM/sacral agenesis critical region of 0.3 Mb near chromosome 6q25.3 by comparing the deleted regions in several of the published cases and included a case with a small interstitial deletion and anteriorly displaced anus [Pirola et al., 1998]. Our own database contains an additional female case with a deletion 6q25.3-qter and an anal atresia without fistula. This case was a terminated pregnancy after the prenatal detection of severe hydrocephaly. In addition to the ARM she also had partial agenesis of the sacrum. Five of the 53 cases with a terminal 6q deletion (9.4%) assembled in the ECARUCA database have an ARM as one of the presenting features. Many of the cases without an ARM are deleted for the critical area described by Titomanlio et al. In addition several recent reviews on 6q deletion syndrome do not report an ARM as a typical feature [Bertini et al., 2006; Elia et al., 2006; Striano et al., 2006].
Chromosome 7
Deletion 7q36
The association of terminal deletions of the long arm of chromosome 7 with ARM is well known and multiple cases have been described [Reynolds et al., 1984; Zackowski et al., 1990; Masuno et al., 1996; Cook et al., 1998; Wang et al., 1999]. This association can be attributed to deletion of one copy of the HLXB9 gene, a homeobox gene implicated in the Currarino triad [Ross et al., 1998]. The full Currarino triad is characterized by an ARM, sacral agenesis, a presacral mass (teratoma) and anterior meningocele. Currarino syndrome follows autosomal dominant inheritance with variable expression and reduced penetrance and is caused by haploinsufficiency for the HLXB9 gene [Lynch et al., 2000]. Even within families, symptoms can vary from the complete triad to only minor sacral anomalies and constipation. As expected patients with 7q terminal deletions that include the HLXB9 gene show the same variation in expression [Horn et al., 2004]. Of the 83 cases with a 7qter deletion that include HLXB9 sampled in the ECARUCA database seven show an anorectal malformation and an additional two have an anteriorly displaced anus. Thirteen have an abnormal sacrum and three have a presacral teratoma. This again shows the variable expression and reduced penetrance. Patients with mutations of the HLXB9 gene similarly show features of Currarino syndrome, along with variable expression and reduced penetrance. However, as can be expected, additional features, especially developmental delay, are present in the patients with a deletion [Cretolle et al., 2008]. This is most likely due to the effect of contiguous genes.
Another important gene with a potential role in anorectal development located in the same region is the Sonic Hedgehog gene, SHH. In humans mutations in SHH are known to cause holoprosencephaly [Roessler et al., 1996]. In humans no mutations have been identified in patients with ARM. However, mice with SHH null mutations and mice with mutations in two other genes from the Sonic Hedgehog signaling pathway (Gli2 and Gli3) show abnormal anorectal development [Mo et al., 2001]. Similarly hedgehog signaling has been shown to be essential for cloacal development in zebrafish [Parkin et al., 2009]. It is currently unknown if deletion of the SHH gene contributes to the presence of anorectal abnormalities in patients with 7q36 deletions.
Chromosome 9
Deletion 9p
Two cases of a distal 9p deletion and anal atresia have been reported [Muroya et al., 2000; Fujimoto et al., 2004]. These two Japanese cases are full cousins and result from a familial translocation t(9;16)(p23;q22). One additional case has an anterior displaced anus [Fujimoto et al., 2004]. The ECARUCA database has 101 cases of distal 9p deletion. Only one of these has an anal atresia but 9 others had an abnormal position of the anus. Except for one patient all of these had an imbalance with an additional duplication of a second chromosome.
Trisomy 9
Three cases of mosaic or non-mosaic trisomy 9 and anorectal abnormalities have been published. One case with a full trisomy 9 had anal atresia [Delicado et al., 1985]. A second case with full trisomy 9 had a hypoplastic anus and genital anomalies [Cantu et al., 1996]. The third case had a mosaic trisomy and a stenotic anus [Saneto et al., 1998]. Two additional cases have abnormal placement of the anus [Anneren and Sedin, 1981; Diaz-Mares et al., 1990]. The ECARUCA database has 50 cases of either mosaic or non-mosaic trisomy 9. Five of these (all from the previous publications mentioned above) have an anorectal abnormality. Only one had anal atresia, the others showed anal stenosis or anteriorly displaced anus.
Chromosome 10
Deletion 10q25
Five cases with an ARM and a deletion of the terminal part of chromosome 10q have been published [Wegner et al., 1981; Wulfsberg et al., 1989; Tsukuda et al., 1996; Maruyama et al., 2001]. An additional two cases showed an anteriorly displaced anus [Lewandowski et al., 1978; Brusnicky et al., 1986]. In three of these cases there was a pure 10q deletion; the others, in addition, had an imbalance of a second chromosome. Early reports suggest that anal anomalies are associated with larger more proximal deletions that include 10q25 [Brusnicky et al., 1986; Wulfsberg et al., 1989], but two other cases with an ARM have more distal breakpoints [Tsukuda et al., 1996; Maruyama et al., 2001]. Our own series also includes one female patient with a deletion 10q26-qter and an ARM. Many other patients with a 10q deletion without an ARM do have genital anomalies, with undescended testis present in almost all males with this deletion. Other patients have a micropenis, bifid scrotum, hypospadias or ambiguous genitals in males and hypoplastic labia majora in females [Waggoner et al., 1999; Scigliano et al., 2004; Courtens et al., 2006]. Ogata et al. [2000] performed deletion mapping in 10 patients and suggest a gene for urogenital development on distal 10q around marker D10S1248 (10q26.3, 130,882,364-131,082,786, UCSC genome browser Human, March 2006).
Chromosome 11
Deletion 11q24
Several cases of monosomy 11q (Jacobsen syndrome) and an ARM have been published [Hatanaka et al., 1984; Leegte et al., 1999; Puvabanditsin et al., 2001; von Bubnoff et al., 2004]. Several of these in addition had genital abnormalities (hypospadias, undescended testes) [Puvabanditsin et al., 2001; von Bubnoff et al., 2004]. The case presented by Hatanaka et al. also had a duplication of 16q13. The ECARUCA database has 94 cases with deletion 11q syndrome. Only two of these have an ARM. In a review of 110 cases, two patients had anal atresia and one had an anteriorly displaced anus [Grossfeld et al., 2004]. In this review 42% had chronic constipation.
Duplication 11q23
Multiple cases of partial trisomy 11q have been described which also have an ARM. Most of them have an imbalance as the result of a familial t(11;22) translocation which also results in a 22q trisomy. It is uncertain whether both the 11q trisomy and the 22q trisomy contribute to the anorectal phenotype in these patients. A case presented by Smeets et al. [1997] had a similar 11q23-qter duplication as a result of a familial t(11;13)(q23;p13) translocation and one of the six patients from a single family described in this article did have an anal atresia and a micropenis (Patient 4). This patient suggests that the 11q duplication on its own does contribute to the anorectal phenotype.
Chromosome 12
Tetrasomy 12p
The Pallister–Killian syndrome (PKS) is a well known genetic disorder defined by the mosaic presence of tetrasomy for the short arm of chromosome 12 (isochromosome 12p). Multiple cases of PKS and ARM have been described [Pauli et al., 1987; Reynolds et al., 1987; Wenger et al., 1990; McLeod et al., 1991; Tejada et al., 1992; Chiesa et al., 1998; Baglaj et al., 2008]. Baglaj et al. report the presence of an ARM in 15% of the cases. Most cases have a low ARM with either anterior displaced anus or a perineal fistula, but there are reports of cases with ARM without fistula [Pauli et al., 1987; McLeod et al., 1991] or with persistent cloaca [Chiesa et al., 1998]. A total of 58 cases of PKS have been included in the ECARUCA database. Nine of these have anal atresia (15.5%). If an anterior displaced anus is considered as an ARM seven additional cases are present, for a total of 16/58 cases (27.5%). Therefore among all chromosomal anomalies PKS appears to have one of the highest frequencies of anorectal malformations.
Trisomy 12p
Partial trisomy 12p is much rarer than tetrasomy 12p and approximately 40 cases have been described in the medical literature. Two of these have an ARM. One boy with an anal atresia and scrotal fistula had a trisomy for the whole short arm of chromosome 12 as the result of a maternal balanced t(12;14) translocation [Stengel-Rutkowski et al., 1981]. A second male patient, trisomic for the 12p12–q12 region as part of a supernumerary ring chromosome 12, had a “VATER like” phenotype. In addition to an anal atresia with rectourethral fistula he had multiple hemivertebrae, sacral agenesis and a single kidney [Cinti et al., 2001].
Chromosome 13
Deletion 13q32
The phenotype of 13q-deletion is widely variable but well recognized. The spectrum includes intellectual disability, growth retardation, retinoblastoma, renal, heart and brain malformation, anorectal and other gastrointestinal abnormalities, genital abnormalities and limb malformation, especially absent or hypoplastic thumbs, and a recognizable facial phenotype [Allderdice et al., 1969; Niebuhr, 1977; Brown et al., 1993, 1995; Walsh et al., 2001; Kirchhoff et al., 2009; Quelin et al., 2009]. Brown et al. divided the patients into three groups. Patients with an interstitial proximal deletion (proximal to q32) show mild intellectual disability, retinoblastoma and otherwise no major congenital anomalies. Patients with deletions including 13q32 have the most severe phenotype, with severe intellectual disability and multiple congenital anomalies and patients with a more distal deletion (terminal to band 13q32) have severe intellectual disability without major congenital anomalies [Brown et al., 1993, 1995]. This would define a critical region for the major congenital anomalies on 13q32. The 13q-deletion syndrome is the chromosomal deletion syndrome that is most commonly associated with ARM. In an early review on 13q-deletion syndrome [Allderdice et al., 1969] an ARM was reported in 5/23 cases.
ARM and penoscrotal transposition (PST)
Typical for the 13q deletion syndrome is the association of ARM with genital abnormalities, especially in males. Classically these patients show the combination of ARM with penoscrotal transposition [Bartsch et al., 1996; Kuhnle et al., 2000]. PST is a rare congenital abnormality defined by a penis positioned more caudally from its normal suprascrotal position with the shaft embedded somewhere along the scrotum or completely posterior to the scrotum [Parida et al., 1995]. PST can occur as an isolated anomaly or in combination with other congenital defects. In a large series of 53 patients with PST 17 (32%) had extragenital anomalies, five of these had an ARM [Pinke et al., 2001]. In a much smaller series the number of PST cases associated with an ARM was as high as 80% [Parida et al., 1995]. In many other male 13q deletion patients other genital abnormalities are present, with hypospadias, bifid scrotum, and undescended testis. Genital abnormalities have also been described in females [Carmichael et al., 1977; Niebuhr, 1977; Kasyan and Benirschke, 2005]. It was suggested that a critical region for PST, hypospadias and ARM is present on chromosome 13q32.2q34 [Bartsch et al., 1996] and the critical region was further mapped to a ∼9.5 Mb interval of 13q33.3–34 containing 20 annotated genes [Garcia et al., 2006]. An interesting candidate gene in this region is the EFNB2 gene (Ephrin B2). A partial loss of function mutation in this gene has been shown to cause hypospadias in a heterozygous state and imperforate anus/persistent cloaca in homozygous state in mice [Dravis et al., 2004; Yucel et al., 2007]. Many of the features described in the 13q deletion syndrome are also known to be part of the VACTERL association. Walsh et al. [2001] describe a patient that would fully classify as VACTERL association with all six features present with additional penoscrotal transposition and hypospadias. They discuss the literature and conclude that many of the published cases would fulfill the criteria for the VACTERL association. This suggests that it is certainly reasonable to look for chromosome 13 deletions in patients with this association especially when genital abnormalities are present. Guala et al. discuss a case of possible XK aprosencephaly syndrome/Garcia–Lurie syndrome (OMIM 207770) with stenotic anus, genital anomalies and a ring-chromosome 13 with effectively a 13q32 deletion [Guala et al., 1997]. McPherson et al. [2004] reviewed this syndrome and the possibly overlapping Steinfeld syndrome (OMIM 184705) and showed that several cases have significant overlap with the 13q deletion syndrome including anogenital abnormalities. An interesting case with DK phocomelia syndrome (OMIM 223340) and anterior displacement of the anus was shown to have mosaicism for a 13q deletion (del13q12qter), with the deletion only present in fibroblasts [Bamforth and Lin, 1997]. This might suggest that in some cases with a 13q deletion phenotype, with normal karyotype in lymphocytes, additional chromosome studies in fibroblasts would be warranted.
Trisomy 13
In their review of additional anomalies with an ARM, Cuschieri [2002] give a percentage of 0.65% of ARM cases with trisomy 13. In other (smaller) series this association is not mentioned. In the literature on trisomy 13 an ARM is not mentioned as a frequent feature and it is not mentioned as a feature in the “Catalogue of Unbalanced Chromosome Aberrations in Man” [Schinzel, 2001]. It is unknown if this difference reflects a failure to recognize this association in general or an overestimation of the relative frequency of this association in the Cushieri series [Cuschieri, 2002].
Chromosome 16
Trisomy 16
Trisomy 16 is one of the most common autosomal trisomies in spontaneous abortions [reviewed by Benn, 1998]. Anorectal malformations have been reported in several cases with non-mosaic trisomy 16 [Cusick et al., 1995; Yancey et al., 1996; Seller et al., 2004]. In addition at least three cases with apparently confined placental mosaicism (CPM) and an anorectal malformation have been published [der Kalousek et al., 1993; Vaughan et al., 1994; Moore et al., 1997]. In these cases maternal uniparental disomy (UPD) was subsequently identified in the fetuses. One additional ARM case with trisomy 16 mosaicism in amniotic fluid cells and maternal UPD16 has been published [Eggermann et al., 2004]. Benn discusses the possible mechanisms that could explain the congenital abnormalities in trisomy 16 [Benn et al., 1983]. CPM could certainly explain intrauterine growth retardation and poor fetal outcome but is unlikely to explain specific congenital abnormalities. UPD for imprinted genes on chromosome 16 is possible but no imprinted genes have yet been identified on chromosome 16. The rare occurrence of UPD for an autosomal recessive mutation is unlikely to explain multiple cases and could not explain similar abnormalities in full trisomy 16 cases. Benn suggests that the most likely explanation would be occult mosaicism; a direct effect of trisomic cells in the developing embryo leading to anomalies in specific tissues. Using this mechanism, a gain of chromosome 16 material could contribute to the anorectal phenotype in these patients. In support of this hypothesis several cases with a partial duplication of the long arm of chromosome 16 with an ARM have been published [Ridler and McKeown, 1979; Garau et al., 1980; Hatanaka et al., 1984; Houlston et al., 1994].
Proximal deletion 16q
Several patients with ARM and a small interstitial deletion of the proximal 16q region, including the SALL1 gene, were detected by high resolution techniques (array-CGH, Q-PCR) [Elder et al., 1984; Knoblauch et al., 2000; Borozdin et al., 2006; Bardakjian et al., 2009]. Two patients had an ARM with rectoperineal fistula, one had a membranous anal atresia, three had an ectopic anus, and one had anal stenosis. Mutations in this gene cause the Townes–Brocks syndrome (TBS) [Kohlhase et al., 1998]. Classic TBS is characterized by imperforate anus, dysplastic ears with hearing impairment and thumb malformations. This autosomal dominant inherited syndrome shows a strong phenotypic variation. Borozdin et al. [2006] show that patients with microdeletions of SALL1 have a relatively mild phenotype compared to patients with dominant negative point mutations.
Chromosome 18
Trisomy 18
Similar to what has been reported for trisomy 13, Cuschieri et al. also report an incidence of 1.08% for trisomy 18 cases among all cases in the registry with ARM occurring with other defects. A similar percentage is reported by Cho et al. [2001]. Except for one case present in the Nijmegen cohort presented before 1996 [Hassink et al., 1996] our cohort of over 500 ARM cases does not include additional cases of trisomy 18. This may be explained by the fact that our patients are selected from a pediatric surgical department and prenatal or neonatally lethal cases may have been missed. The medical literature on trisomy 18 does not comment on the occurrence of anorectal malformations but in the “Catalogue of Unbalanced Chromosome Aberrations in Man” [Schinzel, 2001] anal atresia, stenosis or cloacal extrophy are reported as rare manifestations of trisomy 18.
Chromosome 21
Trisomy 21
The association of Down syndrome with ARM is well known. Two to five percent of the patients with an ARM have trisomy 21 [Spouge and Baird, 1986; Cho et al., 2001; Cuschieri, 2002], as is also shown in our series. They constitute about 40% of the patients with an ARM and a chromosomal anomaly. In different smaller series of patients with Down syndrome an ARM was present in 0.3–2% [Zlotogora et al., 1989; Urioste and Martinez-Frias, 1991; Torfs et al., 1992; Bianca and Ettore, 2000]. In one large series of 5,581 patients with Down syndrome 50 had an ARM (0.9%) [Kallen et al., 1996]. Over 95% of patients with Down syndrome and an ARM have an imperforate anus without fistula [Black and Sherman, 1989; Torres et al., 1998; Endo et al., 1999]. The incidence of Down syndrome in patients with an ARM without fistula is 35–40% and less than 0.5% in patients with a fistula [Endo et al., 1999].
Chromosome 22
Deletion 22q11.2
The 22q11.2 deletion syndrome (Velocardiofacial syndrome, DiGeorge syndrome) is the most common microdeletion syndrome and a plethora of congenital abnormalities have been described at different frequencies in this syndrome. It is not surprising that several cases of anorectal abnormalities have been described among patients with this deletion [Conley et al., 1979; Worthington et al., 1997; Worthington et al., 1997; Yamagishi et al., 1998]. The variability of the clinical expression is very clearly shown by the presence of an ARM in only one of a monozygous twin pair described by Yamagishi et al. [1998]. The ECARUCA database contains 138 cases of the 22q11.2 deletion syndrome, with four of them having an ARM, two with anal atresia and two with anal stenosis.
Duplication 22q
Multiple published cases with incomplete trisomy for chromosomes 22 and 11, due to a familial 11/22 translocation with 3:1 meiotic disjunction have an ARM. They have either anal atresia with or without fistula, anterior anal placement or anal stenosis. In different reviews the number of patients with an ARM ranges from 15% to 30% [Fraccaro et al., 1980; Zackai and Emanuel, 1980; Schinzel et al., 1981b; Iselius et al., 1983]. Again in many cases, especially in males, this is accompanied by genital anomalies; cryptorchidism and micropenis are present in >75% of males with this imbalance [Fraccaro et al., 1980; Zackai and Emanuel, 1980]. Patients with this imbalance show considerable overlap in clinical features with both trisomy 11(q23-qter) and trisomy 22. Genital anomalies would fit better with 11q trisomy, while an ARM would fit better with gain in chromosome 22q [Schinzel et al., 1981b].
Trisomy 22
Several cases with either full or mosaic trisomy 22 have been shown to have anorectal malformations [Iselius and Faxelius, 1978; Schinzel, 1981; Slater et al., 1993]. Both ARM with fistula [Iselius and Faxelius, 1978] and without fistula [Schinzel, 1981], have been described.
Tetrasomy 22pter-q11
Several authors discuss the clinical overlap and differences between cases with incomplete trisomy for chromosomes 22 and 11, due to a familial 11/22 translocation with 3:1 meiotic disjunction and cases of Cat eye syndrome (CES, tetrasomy 22pter-q11) [Fraccaro et al., 1980; Schinzel et al., 1981a]. CES refers to a syndrome of ocular colobomas, preauricular abnormalities, anal atresia and other congenital abnormalities with mild to moderate mental delay. It is considered to be the phenotype of a triplication of the proximal part of the long arm of chromosome 22, usually resulting from a supernumerary marker chromosome. The preauricular abnormalities seem to be pathognomonic but anorectal malformations are also present in the majority of the patients. In the review by Schinzel et al. [1981a] an ARM was present in 22/34 patients, almost always anal atresia with fistula. Five out of six patients described by Liehr had anal atresia with fistula and the sixth had anal stenosis [Liehr et al., 1992]. This makes CES the chromosomal abnormality with the highest incidence of ARM.
DISCUSSION
We have reviewed the medical literature on chromosomal anomalies in patients with anorectal malformation. We do not presume that this review is in any way complete, and, although we have tried to study the literature methodically, we have likely missed multiple cases. However we do think that this review is representative for the occurrence of chromosomal abnormalities in ARM. Excluding trisomy 21, we have found a total of 114 cases of a chromosomal anomaly with ARM, 48 females, and 66 males. Forty-six patients had an isolated chromosomal loss (deletion), 10 had the combination of a deletion on one chromosome and a duplication on another. Fifty-eight patients had a gain, including isolated duplications in 10 cases, trisomy (other than 13, 18 or 21) in 13 cases, tetrasomy in 26 cases and double duplications in nine patients. All reviewed cases are summarized in Table II.
The most frequent chromosomal abnormalities reported are terminal deletion of chromosome 13q in 20 cases, Cat eye syndrome (tetrasomy 22pter-q11) in 19 cases and an unbalanced t(11;22) with a gain of 11q23qter and 22q11.2qter in 8 cases. Other relatively frequent disorders are Pallister–Killian syndrome (PKS, tetrasomy 12p) and a terminal deletion of 10q, with six, and five cases, respectively.
We have looked for differences in the type of ARM in the patients with chromosomal abnormalities. It has previously been suggested that in patients with a chromosomal anomaly anal atresia without fistula is more frequent [Cuschieri, 2002]. This can largely be accounted for by the patients with Down syndrome. They constitute between 30% and 40% of all patients with an ARM and a chromosomal anomaly and in 95% of these a low ARM without fistula is seen. If we exclude Down syndrome, the proportions are different. Of the 114 cases only 6 are reported to have no fistula and 47 have a fistula. In 58 cases it is not mentioned if there is a fistula are not. They are usually reported as imperforate anus or anal atresia without specifying the type of atresia. This might mean they all have no fistula, which would indeed indicate a high frequency of ARM without fistula in this cohort. However, we do think it is more likely that in these latter cases the lack of report of a fistula is mostly due to a failure to describe the full details of the anorectal malformation. The main reason for publication of these reports was not to describe the surgical details but to describe the chromosomal anomaly. In most cases an ARM was just one of many symptoms in the patient. There are no significant differences between the occurrence of the different types of ARM and either a chromosomal loss or gain. Looking at specific chromosomal abnormalities the most striking finding is that in almost all cases of cat-eye syndrome ARM with fistula is reported; 15 out of 19 have a fistula, while in the other four the type of atresia is not specified.
Genital abnormalities are frequent in the group of patients with an ARM and a chromosomal abnormality; 36 out of 66 males (55%) and 6 out of 48 females (12.5%) are reported to have additional genital abnormalities. Among all patients with an ARM these percentages are much lower, 26% and 5% respectively in males and females [Metts et al., 1997]. In males especially cryptorchidism, hypospadias, bifid scrotum/PST, and micropenis are frequent. The combination of ARM with genital abnormalities is particularly frequent in patients with a terminal 13q deletion, reported in 14/20 cases (13 out of 15 males). Also in the terminal 10q deletion syndrome, 11q deletion syndrome (Jacobsen syndrome) and Pallister–Killian syndrome this combination is frequent. It has previously been suggested that candidate genes involved in anorectal and genital development are located on chromosome 13q [Bartsch et al., 1996; Garcia et al., 2006] and chromosome 10q [Ogata et al., 2000]. The increased frequency of ARM with genital abnormalities in patients with chromosomal aberrations does suggest a more significant contribution of genetic factors in this combination compared to patients with an ARM without genital abnormalities. It would also suggest that testing for chromosomal abnormalities in patients with an ARM with genital abnormalities is indicated.
Almost all cases reviewed here have had microscopically visible aberrations. In spite of the increased use of MLPA and array based techniques in the study for copy number variations (CNVs), there are few reports of patients with chromosomal microdeletions or duplications. Several patients with a 22q11.2 deletion detected by FISH have been described as discussed above. When we extended our search using terms that might be used in literature to describe smaller CNVs, we found only a small number of additional cases with an ARM. Several patients had a proximal 16q deletion including SALL1 [Knoblauch et al., 2000; Borozdin et al., 2006; Bardakjian et al., 2009] and they were all missed in our initial search. An additional patient had a submicroscopic 16q24 deletion [Stankiewicz et al., 2009]. One patient had a de novo (balanced) translocation t(6;17)(p21.31;q11.2) leading to a 2 bp deletion on the derivative chromosome 6 and a 7 bp duplication on the derivative chromosome 17 and had an ARM and hypospadias [Mansouri et al., 2006]. Two patients with a small gain on 18q were recently described. One had a gain of chromosome 18p11.21–q12.1 [Schramm et al., 2011]. The other had a small mosaic gain of 18q10–q11.2 [van der Veeken et al., 2010]. No larger study has been performed on the occurrence of CNVs, not detected by conventional karyotyping, in patients with an ARM with additional congenital abnormalities. We suggest that studying a larger cohort of complex ARM cases is likely to detect additional CNVs that might give additional information on chromosomal regions and genes involved in hindgut development.




