Hedgehog signaling update†‡
This article titled “Hedgehog Signaling Update: The First Robert J. Gorlin Lecture” is published in honor of Tom Shepard's Festschrift in Seattle, Washington.
How to cite this article: Cohen MM Jr. 2010. Hedgehog signaling update. Am J Med Genet Part A 152A:1875–1914.
Abstract
In vertebrate hedgehog signaling, hedgehog ligands are processed to become bilipidated and then multimerize, which allows them to leave the signaling cell via Dispatched 1 and become transported via glypicans and megalin to the responding cells. Hedgehog then interacts with a complex of Patched 1 and Cdo/Boc, which activates endocytic Smoothened to the cilium. Patched 1 regulates the activity of Smoothened (1) via Vitamin D3, which inhibits Smoothened in the absence of hedgehog ligand or (2) via oxysterols, which activate Smoothened in the presence of hedgehog ligand. Hedgehog ligands also interact with Hip1, Patched 2, and Gas1, which regulate the range as well as the level of hedgehog signaling. In vertebrates, Smoothened is shortened at its C-terminal end and lacks most of the phosphorylation sites of importance in Drosophila. Cos2, also of importance in Drosophila, plays no role in mammalian transduction, nor do its homologs Kif7 and Kif27. The cilium may provide a function analogous to that of Cos2 by linking Smoothened to the modulation of Gli transcription factors. Disorders associated with the hedgehog signaling network follow, including nevoid basal cell carcinoma syndrome, holoprosencephaly, Smith–Lemli–Opitz syndrome, Greig cephalopolysyndactyly syndrome, Pallister–Hall syndrome, Carpenter syndrome, and Rubinstein–Taybi syndrome. © 2010 Wiley-Liss, Inc.
INTRODUCTION
On September 19, 2007, I was greatly honored to be invited to the University of Minnesota to deliver the First Robert J. Gorlin Lecture at Pediatrics Grand Rounds. As appropriate for a Pediatrics Grand Rounds, I began with clinical cases and then moved on to discuss vertebrate hedgehog signaling on the cilium. This article permits an extensive treatment of hedgehog signaling with references.
IMPORTANT NOMENCLATURE ISSUES
Two issues of nomenclature in hedgehog signaling are so important that they are considered first. Although the term “hedgehog signaling pathway” is most commonly used, the pathway is complex, and strikingly different phenotypes result from various types of mutations. For example, phenotypes include, among others, holoprosencephaly, Gorlin syndrome (nevoid basal cell carcinoma syndrome), some isolated basal cell carcinomas, medulloblastoma, Greig cephalopolysyndactyly, Pallister–Hall syndrome, Rubinstein–Taybi syndrome, and Carpenter syndrome (Table I). Elsewhere [Cohen, 2003a], I have used the term “hedgehog signaling network.”
| Conditions | Mutated genes |
|---|---|
| Holoprosencephaly | SHH |
| PTCH1 | |
| GLI2 | |
| DISP1 | |
| SIX3 | |
| Nevoid basal cell carcinoma syndrome | PTCH1 |
| PTCH2 | |
| Greig cephalopolysyndactyly | GLI3 |
| Pallister–Hall syndrome | GLI3 |
| Rubinstein–Taybi syndrome | CBP |
| Carpenter syndrome | RAB32 |
| Nonsyndromic colobomatous microphthalmia | SHH |
| Isolated single maxillary central incisor | SHH |
| Rare missense changes of unknown significance in nonsyndromic cleft lip and palate | PTCH1 |
| Basal cell carcinoma | PTCH1 |
| PTCH2 | |
| SMO | |
| Medulloblastoma | PTCH1 |
| PTCH2 | |
| SMO | |
| SUFU | |
| GLI3 | |
| SHH | |
| Meningioma | PTCH1 |
| Primitive neuroectodermal tumor | PTCH1 |
| Breast cancer | SHH |
| PTCH1 | |
| Squamous cell carcinoma | PTCH1 |
| Trichoepithelioma | PTCH1 |
| Esophageal carcinoma | PTCH1 |
| Fetal rhabdomyoma | PTCH1 |
| Rhabdomyosarcoma | PTCH1 |
| Condition | Comments |
|---|---|
| Other disorders | |
| Intestinal polyposis, cancer, and Gardner syndrome | WNT genes are targets of Hedgehog signaling. Two disorders of WNT signaling mutations in (1) APC or β-Catenin and (2) AXIN2 |
| Multiple exostoses | EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparin sulfate. EXT2 has a 44% protein identity and EXT1 has a 26% identity to tout-velu, which is implicated in heparin sulfate proteoglycans. tout-velu is involved in the transfer of Hedgehog from the signaling cell to the responding cell |
| Smith–Lemli–Opitz syndrome | Autosomal recessive syndrome caused by DHCR7 mutations. About 5–6% of cases have holoprosencephaly. Decreased levels of cellular sterols correlate with diminished responsiveness to the hedgehog signal. There are sufficient levels to produce the cholesterol moiety of activated hedgehog. Instead, smoothened conformation appears to be the target of cholesterol deprivation |
| VACTERL | Shares some features with Pallister–Hall syndrome, including anal atresia and cardiac, renal, and limb anomalies. A murine model of VACTERL suggests that digenic Gli genes may be responsible for murine VACTERL. This hypothesis is testable in humans |
The symbol for the patched gene first introduced in Drosophila was ptc. Later, when Gorlin syndrome was shown to be caused by mutations in PATCHED, a problem in nomenclature was introduced. It is traditional to write the human gene name in upper case, so it would be PTC. However, antedating the discovery of ptc in Drosophila, a human gene PTC had already been postulated [OMIM 171200, Phenylthiocarbamide (PTC) tasting] and more recently identified for the ability to taste phenylthiocarbamide [Kim et al., 2003]. We cannot have the same symbol for two different genes, so I suggested that the symbol for the gene PATCHED should be changed to PTCH [Cohen, 2003a]. To be consistent, the Drosophila gene should be renamed ptch. Human geneticists and most biologists now use PTCH for the human gene. Some biologists refer to the human gene as PTCH, the vertebrate gene as Ptch, but for the Drosophila gene, ptc is usually retained.
OVERVIEW OF HEDGEHOG SIGNALING
In the signaling network, hedgehog ligands are processed to become bilipidated and then multimerize, which allows them to leave the signaling cell via Dispatched and become transported via glypicans and megalin to the responding cell. There, ligand binding to Patched releases Smoothened from an endocytic vesicle to the plasma membrane in Drosophila or to the cilium in vertebrates [Corbit et al., 2005], resulting in downstream signaling (Fig. 1). Several reviews are available [Hooper and Scott, 2005; Huangfu and Anderson, 2006; Wang et al., 2007; Varjosalo and Taipale, 2008]. Although significant progress has been made in understanding functions and mechanisms in hedgehog signaling and several new components have been identified, many problems and some contradictory findings remain to be elucidated.
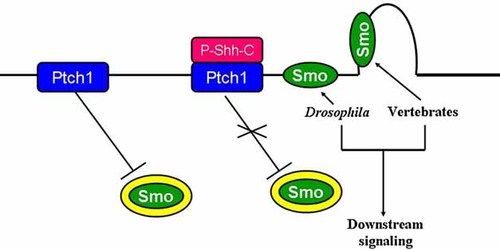
When Sonic hedgehog (P-Shh-C) binds to Patched 1 (Ptch1), Smoothened (Smo) shuttles from an endocytic vesicle to the cilium in vertebrates. For Drosophila, in contrast, when hedgehog (P-Hh-C) binds to Ptch1, Smo shuttles from an endocytic vesicle to the plasma membrane.
HEDGEHOG GENES
Unlike the single Hedgehog gene found in Drosophila (Hh), three Hedgehog genes have been found in vertebrates. These include Desert hedgehog (Dhh), Indian hedgehog (Ihh), and Sonic hedgehog (Shh). Dhh is most closely related to Hh. Ihh and Shh are more closely related to each other and represent more recent gene duplication. Further duplication within the Ihh and Shh classes has occurred in teleost fishes [Ingham and McMahon, 2001]. Five homologs are found in zebrafish (zebrafish website: http://zfin.org) and three of them play important roles in embryonic patterning: Sonic hedgehog (Shh), Echidna hedgehog (Ehh), and Tiggywinkle hedgehog (Twhh) [Ekker et al., 1995; Currie and Ingham, 1996]. Iguana encodes a zinc-finger protein with coiled-coil domains essential for hedgehog signaling transduction in zebrafish embryos. The gene product acts downstream of the Smoothened protein to modulate Gli activity in the somites of the developing embryo [Wolff et al., 2007].
The three mammalian hedgehogs have specialized functions. Dhh expression is mostly restricted to the gonads, including Sertoli cells in the testes, granula cells in the ovaries, but also in the formation of neural sheaths. Ihh is expressed in the primitive endoderm and in prehypertrophic chondrocytes in the growth plates during endochondral bone formation. Shh is broadly expressed in many mammalian tissues. During early embryogenesis, Shh transcripts are found in the notochord, ventral neural plate, and in the patterning of left–right and dorsoventral axes in the embryo. Shh is expressed in the zone of polarizing activity (ZPA) of the limb buds and in the patterning the distal elements of the limbs. Shh transcripts are also expressed in the pituitary gland, development of the eye, olfactory pathway formation, dental development, many gut-derived organs, the heart, the lungs, the prostate gland, and in smooth muscle regulation [Ingham and McMahon, 2001; Cohen, 2003a; McMahon et al., 2003; Varjosalo and Taipale, 2008].
LIPID ADDUCTS ATTACHED TO HEDGEHOG LIGAND
An active Hedgehog ligand is generated with a C-terminal cholesterol moiety followed by an N-terminal palmitate (Fig. 2), thus becoming double lipid-modified [Porter et al., 1996; Pepinsky et al., 1998; Chamoun et al., 2001]. Specifically, an autoprocessing reaction is followed by palmitoylation. In the autoprocessing reaction, SHH has an N-terminal signaling domain (SHH-N) and a C-terminal catalytic domain (SHH-C) which causes autocleavage of the protein, producing an ester-linked cholesterol moiety at the C-terminal end of SHH-N. The autocleavage proceeds via a thioester intermediate that undergoes a nucleophilic attack by cholesterol, resulting in a covalently linked cholesterol adduct and activation of the SHH-N signaling portion. The SHH-C catalytic portion diffuses away [Porter et al., 1996]. Following the autoprocessing reaction, palmitoylation of the most amino terminal cysteine of the SHH-N signaling portion takes place, requiring the action of Skinny hedgehog (Ski) acyltransferase [Pepinsky et al., 1998; Chamoun et al., 2001] (Fig. 2).
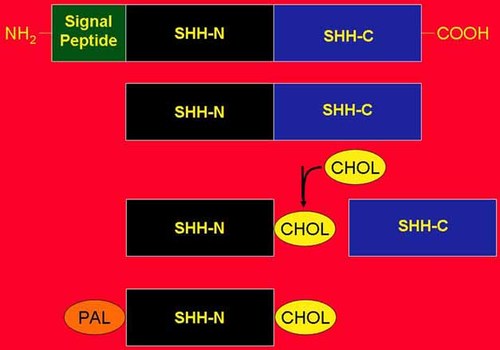
Sonic Hedgehog (SHH) has an N-terminal signaling domain (SHH-N) and a C-terminal catalytic domain (SHH-C) that causes autocleavage of the protein, resulting in an esther-linked cholesterol moiety (CHOL) at the carboxy-terminal end of the signaling domain; the catalytic portion diffuses away. Following the addition of the cholesterol adduct, the action of Skinny hedgehog (Ski) acyltransferase adds an amide-linked palmitate (PAL) to the N-terminal end of the signaling domain. In the responding cell, this bilipidated Sonic Hedgehog (PAL-SHH-CHOL) becomes the ligand for PATCHED 1 (PTCH1). From Cohen [2003a].
The role of lipid adducts is also important in two other ligands: Wnt and Spitz (Drosophila TGFα). Both have an N-terminal palmitate added during biosynthesis in the endoplasmic reticulum. Palmitoylation to activate these two ligands, like Hedgehog ligand, also requires the action of Skinny hedgehog (Ski) acyltransferase [Wendler et al., 2006].
HEDGEHOG SIGNALS
Studies of the C-Terminal Cholesterol Moiety
Many studies have been carried out with contradictory results, which suggest that the C-terminal cholesterol moiety either aids or hinders hedgehog transport [Wang et al., 2007; Varjosalo and Taipale, 2008]. Early studies of the Drosophila imaginal discs suggested that the addition of cholesterol restricts the range of hedgehog movement, but its absence extends the range of movement [Porter et al., 1996; Burke et al., 1999]. Mouse limb studies have suggested that the cholesterol moiety is critical for long range signaling [Lewis et al., 2001], although a more recent study suggests that cholesterol modification restricts the spread of the Shh gradient in the limb bud [Li et al., 2006]. Studies in which both the C-terminal cholesterol moiety and the N-terminal palmitate are both absent, hedgehog can escape the producing cell without even requiring Dispatched [Mann and Beachy, 2004]. Resolving such contradictory findings will be critical in understanding the precise function of cholesterol in the trafficking and activity of hedgehog protein [Wang et al., 2007].
In Drosophila, hedgehog co-purifies with lipoprotein particles. These lipoproteins are necessary for hedgehog signaling [Panakova et al., 2005], suggesting that lipophilic modifications, such as cholesterol serve, at least in part, to localize Shh to certain areas of the plasma membrane critical for proper hedgehog trafficking and activity [Wang et al., 2007].
Enhancer Element Control of Shh Tissue Expression
The action of multiple enhancer elements acting independently control the transcription of Shh in different tissues. “Two independent enhancers—Shh floor plate enhancer 1 (SFPE1) and SFPE2, located at −8 kb and in intron 2, respectively—act to direct reporter expression to the floor plate of the hindbrain and spinal cord. A third element in intron 2, Shh brain enhancer (SBE1), directs reporter expression to the ventral midbrain and caudal diencephalon. The more distal elements, SBE2, SBE3, and SBE4, which are located >400 kb upstream of Shh transcription start site (TSS) drive reporter expression in the ventral forebrain. The combined activity of these enhancers appears to cover all regions of Shh transcription along the anteroposterior axis of the mouse neural tube” [Varjosalo and Taipale, 2008].
“The enhancer controlled Shh expression in the zone of polarizing activity (ZPA) of limb buds, mammal and fish conserved sequence 1 (MFCS1), is located even further upstream of the start site, at −1 Mb in intron 5 of the Lmbr1 gene. This element is the only enhancer in Shh that has been analyzed also by loss-of-function studies, which demonstrate that MFCS1 is necessary for Shh expression in the mouse ZPA. In humans, germline mutations within the conserved MFCS1 element can cause limb malformations characterized by preaxial polydactyly. Although many enhancers that drive Shh expression have been identified, very little is known about the specific transcription factors that control their activity” [Varjosalo and Taipale, 2008].
Different Effects Produced by Hedgehog Signals
Hedgehog signals can produce different effects. In Drosophila, Hh can act as an on/off switch that regulates only adjacent cells as, for example, in the dorsal embryonic ectoderm [Ingham and Hidalgo, 1993; Thérond et al., 1999]. Hh can also act as a short-range morphogen that spreads more than 10–20 cell diameters to control several different fates as a function of its concentration. For example, a model for understanding the short-range morphogen activity in the Drosophila wing disc is based on the bifunctional transcriptional regulator, Cubitus interruptus (Ci) [Strigini and Cohen, 1997]. In the absence of Hh, Ci becomes truncated, which represses target genes. Low levels of Hh block the truncation of Ci, which causes derepression of some target genes, but activated Ci is not made. At higher levels of Hh, Ci is fully converted to the activator form, and with the aid of Fused (Fu) moves to the cell nuclei [Méthot and Basler, 2000; Hooper and Scott, 2005].
Furthermore, Hedgehog can act as a long-range morphogen that spreads over many cell diameters as a function of different concentrations that specify a series of cell fates. For example, in the mouse neural tube, it has been suggested that different Shh levels are directly translated into different ratios of Gli activator/Gli repressor, which control cell fate [Jacob and Briscoe, 2003; Stamataki et al., 2005; Huangfu and Anderson, 2006].
PATCHED 1, PATCHED 2, AND DISPATCHED 1
The structures of Patched 1, Patched 2, and Dispatched 1 are diagramed in Figure 3. Two Patched homologs (Ptch1, Ptch2) are found in zebrafish, mice, and humans. Ptch1 is the major receptor in embryonic development [Goodrich et al., 1997; Cohen, 2003a; Wolff et al., 2003], and in zebrafish, Ptch2 mutants have a relatively mild phenotype [Koudijs et al., 2005].
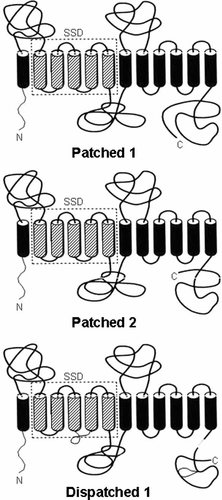
Patched 1, Patched 2, and Dispatched 1 are 12-pass transmembrane proteins. All three have amino- and carboxy-termini located intracellularly. Patched 1 and Patched 2 have different amino- and carboxy-terminal domains, including the absence of 150 amino acid residues from the C-terminal end of Patched 2. Two large extracellular loops on all three proteins determine where hedgehog binding takes place. The sterol sensing domain (SSD) is shown with hatched cylinders enclosed within a rectangle with dashed lines. From Cohen [2003a]. See text.
In humans, PTCH1 is a tumor suppressor gene that maps to 9q22.3 and encodes a 1,500 amino acid glycoprotein with 12 hydrophobic membrane-spanning domains, intracellular amino- and carboxy-terminal regions, and two large extracellular loops (Fig. 3) where Hedgehog ligand binding occurs [Hooper and Scott, 1989; Marigo et al., 1996].
Experimental studies have shown that Ptch1 has dual roles in sequestering and transducing Hh [Chen and Struhl, 1996]. When the second large extracellular loop, essential for ligand binding, is deleted by a Ptch1 mutation, Hh binding to Ptch1 cannot occur, and Ptch1's repression of Smo is unaffected [Briscoe et al., 2001]. When a C-terminal truncation is caused by a Ptch1 mutation, Ptch1 can no longer repress Smo, but Hh binding to Ptch is unaffected [Johnson et al., 2000].
Teratogenic doses of retinoic acid inhibit the expression of both Shh and Ptch1 in the craniofacial primordia of chick embryos [Helms et al., 1997]. These findings are of particular interest because in humans SHH mutations [Roessler et al., 1996, 1997; Nanni et al., 1999] and some PTCH1 missense mutations [Ming et al., 2002; Ribeiro et al., 2006b] have been shown to cause holoprosencephaly. Ptch1 is a lipoprotein receptor, which internalizes lipophosphorins into an endocytic compartment in an Hh-independent manner and physically interacts with those lipophorins. Ptch1 can affect intracellular lipid homeostasis in cells of the wing imaginal disk, but internalization of lipophorins does not play a major role in Hh signal transduction, but does in Hh gradient formation [Callejo et al., 2008].
PTCH1 mutations have been identified in the nevoid basal cell carcinoma syndrome (Gorlin syndrome), some isolated basal cell carcinomas, medulloblastoma, meningioma, neuroectodermal tumor, carcinoma of the breast, esophageal carcinoma, squamous cell carcinoma, and trichoepithelioma [Cohen, 2003a] (Table I). Most mutations result in protein truncation [Cohen, 1999, 2003a], but as indicated above, some PTCH1 missense mutations have been identified in holoprosencephaly [Ming et al., 2002; Ribeiro et al., 2006b].
In humans, PTCH2 maps to 1p32–p34 and encodes a 1,204 amino acid protein. PTCH2 has a 54% overall identity to PTCH1 and a 90% identity to Ptch2 in mice [Carpenter et al., 1998]. The PTCH2 protein contains 12 transmembrane domains and two large extracellular loops. However, both amino- and carboxy-termini are different from those in PTCH1, including absence of 150 amino acid residues in the C-terminal domain (CTD) (Fig. 3) [Cohen, 2003a].
Ptch1 and Ptch2 are differentially expressed during development of the epidermis, suggesting that the two proteins have different functions [Motoyama et al., 1998; Smyth et al., 1999]. Null mice (Ptch2−/−) are viable, but Ptch2−/− adult mice develop alopecia, ulceration, and epidermal hyperplasia, suggesting that normal Ptch2 function is required for adult skin homeostasis [Nieuwenhuis et al., 2006].
Rare human PTCH2 mutations have been reported in medulloblastoma, basal cell carcinoma [Smyth et al., 1999], and in one family with nevoid basal cell carcinoma syndrome [Fan et al., 2008] (Table I).
In humans, DISPATCHED1 (DISP1) maps to 1q42 and encodes a 1,401 amino acid protein [Ma et al., 2002]. Like PTCH1 and PTCH2, DISP1 is a 12-pass transmembrane protein with intracellular amino- and carboxy-terminal regions, and two extracelluar loops (Fig. 3) [Cohen, 2003a].
Disp1 is not required for lipid attachment to Hh, but releases cholesterol-modified Hh from its tether to the plasma membrane of the signaling cell and permits transport to the responding cell [Burke et al., 1999; Kawakami et al., 2002; Tian et al., 2004]. Disp1, highly conserved in Drosophila, zebrafish, and mice, is essential for the secretion of lipid-modified Hh proteins [Burke et al., 1999; Kawakami et al., 2002; Nakano et al., 2004; Tian et al., 2004]. It has been shown in vertebrates that palmitoylation (Fig. 2) is required for the production of a soluble multimeric Hh protein complex and long range signaling [Chen et al., 2004]. The finding of a highly active multimeric complex of lipid-modified Hh ligands [Zeng et al., 2001] suggests that perhaps Disp1, rather than functioning in the general release of ligands, may function instead in the formation and/or release of an active fraction composed of Hh oligomers [Tian et al., 2004].
There are two types of Disp: Disp1, which is essential for the secretion of lipid-modified Hh proteins in zebrafish and Disp2, which is necessary for normal embryonic development in zebrafish, but is dispensable for Hh signaling [Nakano et al., 2004]. The latter is consistent with the failure of the murine Disp2 ortholog to rescue the Disp1 mutation or potentiate the secretion of Shh by tissue culture cells [Ma et al., 2002].
STEROL SENSING DOMAIN
PTCH1, PTCH2, and DISP1 each have a sterol sensing domain (SSD) consisting of approximately 180 amino acids that make up five membrane spanning domains (Fig. 3). Other SSD-containing proteins such as NPC1, HMGCR, SCAP, and DHCR7 play key roles in different aspects of cholesterol homeostasis or cholesterol-linked signaling. They share common properties such as rapid trafficking between organelles, cargo transport, and modification of their activity by sterol and/or lipoprotein concentrations [Kuwabara and Labouesse, 2002].
A subset of SSD, including Ptch1, Ptch2, and Disp, shows sequence similarities to the prokaryotic RND permease superfamily [Tseng et al., 1999]. These proteins are known to function as proton-driven anteporters. They have a conserved GxxxD motif in the middle of TM4 and an aspartic acid residue within this motif, which is important as a protein-binding site. For example, Disp has three aspartic acid residues, suggesting the possibility that Disp and bacterial proteins might act by a similar mechanism [Ma et al., 2002; Taipale et al., 2002].
The SSD of Ptch1 plays no role in the autocleavage and palmitoylation of Shh. Deletion of the C. elegans gene Ce-ptch1 disrupts cytokinesis, suggesting that Ptch1 may have other functions besides inhibiting Smo and sequestering Hh [Kuwabara et al., 2000]. It has also been suggested that the SSD of Disp1 may play some role in the regulation of bilipidated Shh export from the signaling cell [Tian et al., 2004]. However, the specific function of the SSD in Ptch1 and Ptch2 remains unknown to date. It is at least conceivable that evolution has resulted in retention of the SSD as a vestige in these particular receptors that may no longer have a function.
SMOOTHENED
Because Smoothened (Smo, SMO) transduces different concentrations of Hedgehog ligand, its regulation and its function are complex. SMO has seven hydrophobic membrane-spanning domains, an extracellular amino-terminal region, and an intracellular carboxy-terminal region. SMO has some similarities to G protein-coupled receptors, but strongly resembles the Frizzled (Fz) family of receptors for Wnt signaling [Alcedo et al., 1996; van den Heuvel and Ingham, 1996]. A cysteine-rich domain (CRD) is found at the N-terminal end in both Smo and Fz. The CRD is essential for Fz binding to the Wnt family of ligands, but its function in Smo is unknown. CRD mutations in Drosophila disrupt Smo activity [Alcedo et al., 2000; Nakano et al., 2004], whereas CRD deletion in mammalian cells does not affect the activity of overexpressed Smo [Murone et al., 1999; Taipale et al., 2002].
Sequence comparisons of Smo in Drosophila and vertebrates show that the seven transmembrane domains are relatively conserved across species, but significant divergence occurs in the CTD (Fig. 4), suggesting that Smo acts differently in Drosophila than in the vertebrates. Study of the C-terminal domain shows that only 180 amino acids in the juxtamembrane region are conserved in Drosophila and the vertebrates. The 400 amino acids lying more C-terminally in Drosophila have only short patches of homology between Drosophila and the vertebrates [Huangfu and Anderson, 2006]. In Drosophila, 26 serine/threonine residues have been identified in the Smo C-terminal tail; these include three or four PKA sites, seven or eight CKI sites, and one possible GSK3 site. This collective phosphorylation of multiple serine/threonine residues can modulate Smo activity and its capacity to transduce the hedgehog signal. Most of these phosphorylation sites are not conserved in mammalian Smo [Zhang et al., 2004].
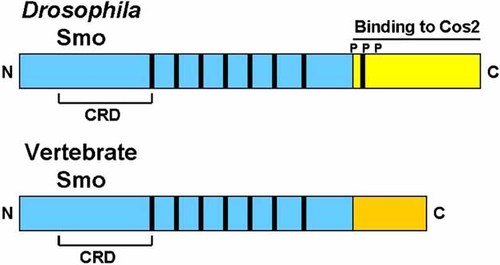
Comparison of Smoothened (Smo) in Drosophila and in the vertebrates. Smo is a 7-pass transmembrane protein represented by seven black lines. CRD represents a cysteine-rich domain. Note divergence in the yellow and orange areas which represent the intracellular C-terminal domain, with dramatic shortening of the vertebrate domain. Drosophila Smo can bind to Costal 2 (Cos2), but vertebrate Smo cannot. In Drosophila, the collective phosphorylation of multiple serine/threonine residues can modulate Smo activity and its capacity to transduce the hedgehog signal. Most of these phosphorylation sites are not conserved in mammalian Smo. Modified and adapted from Huangfu and Anderson 2006. See text.
Most membrane-bound receptors activate downstream signaling on ligand binding. In contrast, Patched is repressed by its Hedgehog ligand, freeing Smoothened for downstream signaling [Ingham et al., 2000; Ingham and McMahon, 2001]. Patched and Smoothened do not interact physically in transducing hedgehog signals. Smoothened shuttles from an endocytic vesicle to the plasma membrane in Drosophila and to the cilium in vertebrates in response to Hedgehog ligand (Fig. 1). Patched acts catalytically instead of stoichiometrically to suppress the activity of Smoothened. For example, a Patched/Smoothened ratio of 1:45 can suppress nearly 80% of Smoothened activity, which indicates regulation possibly by a small molecule [Denef et al., 2000; Ingham et al., 2000; Taipale et al., 2002].
Several synthetic molecules can modulate the activity of Smo, including cyclopamine, a hedgehog antagonist, [Chen et al., 2002] and purmophamine, a hedgehog agonist [Sinha and Chen, 2006]. It has been speculated that Ptch1 may function to inhibit Smo via an intermediate small molecule. Ptch1 induces the secretion of Vitamin D3, which can act directly to inhibit Smo [Bijlsma et al., 2006]. Oxysterols, downstream of Vitamin D3 in the cholesterol biosynthetic pathway, activate Smo [Corcoran and Scott, 2006]. These apparent contradictions may be explained by the presence or absence of Shh ligand. The presence of Shh ligand binding may result in increased secretion and transport of oxysterols. Absence of Shh ligand may result in high levels of Vitamin D3 [Wang et al., 2007].
Sterol depletion, but with still enough for cholesterol moiety attachment to hedgehog, may affect the activity of Smo [Cooper et al., 2003]. Ptch1, which is structurally related to transporter and pump proteins, may pump endogenous sterols away from Smo, inhibiting its action. In the presence of hedgehog binding, or with the loss of Ptch1, sterols may bind to Smo and activate it. Figure 5 demonstrates three experimental possibilities. With no sterols, Smo is inactive, whereas with sterols, Smo is active. When sterols and cyclopamine are combined, Smo is inactive because cyclopamine is a hedgehog antagonist [Corcoran and Scott, 2006]. GDC-0449 was used in a clinical trial of patients with advanced or metastatic basal cell carcinomas and showed anti-tumor activity. It inhibited SMO with more potency and better pharmaceutical properties than cyclopamine [Von Hoff et al., 2009].
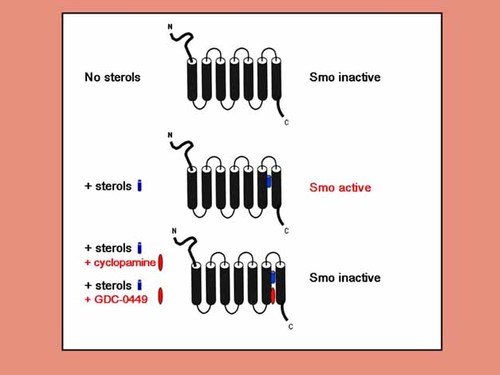
Sterol depletion, but with enough for cholesterol moiety attachment to hedgehog, which affects the activity of Smoothened (Smo). Patched 1 (Ptch1), which is structurally related to transporter and pump proteins, may pump endogenous sterols away from Smo, inhibiting its action. In the presence of hedgehog binding, or with the loss of Ptch1, sterols may bind to Smo and activate it. With no sterols (top), Smo is inactive, whereas with sterols (middle), Smo is active. When sterols and cyclopamine are combined (bottom), Smo is inactive because cyclopamine is a hedgehog antagonist. Modified and adapted from Corcoran and Scott 2006. See text.
SIMILARITIES OF HEDGEHOG AND WNT SIGNALING
Hedgehog signaling and Wnt signaling share a number of similarities and, in fact, invite speculation that they may have evolutionary links. Both have lipid attachments; both have similar receptors; both use protein kinases; both utilize heteromeric complexes in their cytoplasm; and both have extracellular inhibitors and feedback [Taipale and Beachy, 2001; Nusse, 2003; Lum and Beachy, 2004; Mann and Beachy, 2004]. Hedgehog signaling is diagrammed in Figure 6 and Wnt signaling is diagrammed in Figure 7.
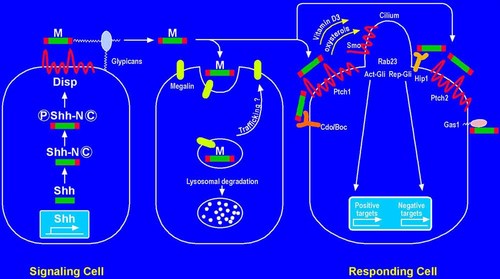
Hedgehog signaling using Shh as a model. In the signaling cell, autoprocessing generates an N-terminal signaling domain with a C-terminal cholesterol moiety (C). Then palmitoylation results in an N-terminal palmitate. The bilipidated hedgehog proteins are trafficked to the cell surface mediated by Dispatched (Disp) as multimers (M). These multimers travel to the responding cell via the interactions of glypicans and megalin, which itself regulates hedgehog protein turnover. In the responding cell, Shh interacts with the Ptch1 and Cdo/Boc, resulting in the activation of Smo, which is shuttled from an endocytic vesicle to the cilium (see Fig. 1) to activate the hedgehog signaling cascade. Ptch1 regulates the activity of Smo via either (1) Vitamin D3, which inhibits Smo in the absence of Shh ligand or (2) oxysterols, which activate Smo in the presence of Shh ligand. Shh ligand also interacts Ptch2, Hip1, and Gas1 to regulate the level of hedgehog signaling. The signaling cascade begun by Smo culminates in the activation of Gli (Act-Gli) or the repression of Gli (Rep-Gli). Act-Gli results in the activation of positive targets and Rep-Gli results in the repression of negative targets (see Target Genes Section in text). Rab23, a negative regulator of hedgehog signaling, acts downstream of Smo, but upstream of Gli2, Gli3, and the intraflagellar transport proteins. Modified and adapted from Cohen [2003a] and Wang et al. 2007.
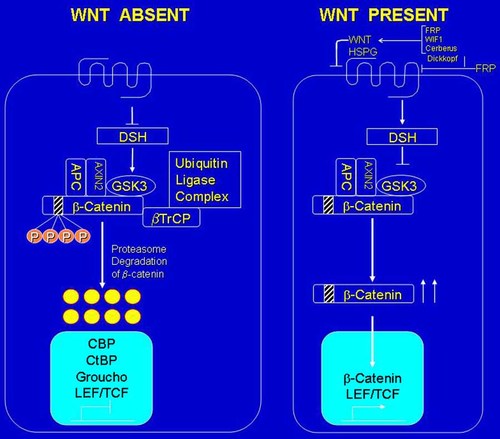
In WNT signaling, a WNT ligand binds to a receptor complex composed of a 7-pass transmembrane Frizzled (Fz) receptor protein and a 1-pass low density lipoprotein receptor-related protein (LRP). The heteromeric complex is composed of 1 of 19 possible Wnt ligands and 1 of 2 possible LRPs. AXIN2, a cytoplasmic component of the Wnt signaling pathway, interacts with the cytoplasmic tail of LRP5 and by extension, LRP6. In the WNT/β-catenin pathway, when WNT is absent, β-catenin is ubiquinated and degraded by the proteasomal pathway, resulting in low levels of β-catenin. Absence of WNT allows DSH to activate GSK3, which then phosphorylates β-catenin followed by its ubiquitinization by βTrCP and proteasomal degradation. This involves a multiprotein destruction complex composed of APC, AXIN2, GSK3, and βTrCP. A LEF/TCF transcription factor together with co-factors CBP, CtBP, and Groucho repress target genes. When WNT is present, DSH inhibits phosphorylation of β-catenin by GSK3. This results in excess β-catenin, which translocates to the nucleus, and together with LEF/TCF upregulates target genes. Regulation of β-catenin is essential for the tumor suppressor effect of APC. This can be circumvented by mutations in either APC or β-catenin, resulting in familial adenomatous polyposis. HSPG is required for optimal signaling. FRP, WIF1, and Cerebus can directly bind to WNT and antagonize its function by interfering with binding to Frizzled. Dickkopf inhibits WNT signaling by binding to the LRP5/LRP6 component of the WNT receptor complex. From Cohen [2003b].
Both hedgehog and Wnt1 proteins have a palmitate amide linkage. Hedgehog gains a palmitate attachment on a conserved cysteine residue at the N-terminal end of the protein. Wnt gains a palmitate at the first conserved cysteine residue (C77). Hedgehog has a cholesterol ester linkage at its C-terminal end attached by an autoprocessing reaction, before palmitoylation, whereas Wnt has no cholesterol moiety [Nusse, 2003; Lum and Beachy, 2004; Mann and Beachy, 2004].
Smoothened (Smo) and Frizzled (Fz)1 are similar structurally. Both are serpentine receptors with seven transmembrane domains and an N-terminal CRD. Smo and Fz are more closely related to each other than they are to others in the family of serpentine receptors [Nusse, 2003].
The mechanisms of activation of Smo and Fz, however, are different from each other. The action of Smo is dependent on repression by Ptch or by hedgehog ligand binding to Ptch, which frees Smo for downstream signaling. The relationship between Ptch and Smo can be modified by a C-terminal Ptch truncation mutation, which releases its inhibition of Smo or by an activating Smo mutation. In contrast, Fz is activated directly by various Wnt, which is an extracellular ligand [Nusse, 2003].
Both hedgehog and Wnt signaling make use of heteromeric complexes in the cytoplasm (Ci, Cos2, Fu, and Sufu in Hh signaling in Drosophila1 and APC, Axin2, GSK3, and β-catenin in Wnt signaling. Both use several related or identical components, such as GSK3, CK1, and Slimb ubiquitin ligase subunit [Lum and Beachy, 2004].
Members of the LDL receptor-related protein family are important in Wnt signaling (Lrp5, Lrp6) and hedgehog signaling (Lrp2). Lrp2, Lrp5, and Lrp6 are long single-pass transmembrane proteins and either Lrp5 or Lrp6 is required for Wnt signaling in addition to a Fz receptor activated by a Wnt ligand with a N-terminal palmitate. The cytoplasmic tail of Lrp5, and by extension, Lrp6, may interact directly with Axin2. Lrp2 encodes megalin, a multi-uptake receptor that regulates diverse components. Perhaps megalin-mediated endocytosis of bilipidated Shh ligand regulates its availability to Ptch by limiting its levels [McCarthy et al., 2002; Nusse, 2003].
Donnai–Barrow syndrome, an autosomal recessive disorder caused by LRP2 mutations, is characterized by facial anomalies, ocular defects, sensorineural hearing loss, and proteinuria [Kantarci et al., 2007]. Absence of megalin in knockout mice (Lrp2−/Lrp−) produces holoprosencephaly [Willnow et al., 1996].
LRP5 mutations are of two general types: (1) autosomal dominant gain-of-function missense mutations resulting in high bone mass phenotypes (osteopetrosis, endosteal hyperostosis, and Van Buchem disease) and (2) autosomal recessive loss-of-function mutations resulting in a low bone mass phenotype (osteoporosis-pseudoglioma syndrome) [Cohen, 2006]. An LRP6 mutation has been identified in ADCAD2 (early coronary artery disease and metabolic syndrome) [Mani et al., 2007].
A heparin sulfate proteoglycans (HSPG) is required for Wnt signaling. In fact, “just a spoonful of sugar helps the signal go down” [Cumberledge and Reichsman, 1997]. In the transport of Hh multimers from the signaling cell across a field of responding cells, HSPGs are implicated, including the heparin sulfate synthesizing enzymes EXT, tout-velu (ttv), brother of tout-velu (botv), and sister of tout-velu (sotv) [Bellaiche et al., 1998; Lin et al., 2000; Bornemann et al., 2004; Han et al., 2004; Koziel et al., 2004].
Wnt antagonists are of two functional classes: (1) secreted Frizzled-related protein (sFRP), which includes the sFRP family, Wnt inhibitory factor 1 (WIF1), and Cerebus; these bind directly to Wnt and alter their ability to bind to the Wnt receptor complex and (2) the Dickkopf family, particularly Dickkopf 1 (Dkk1); they inhibit Wnt signaling by binding to the LRP5/LRP6 component of the Wnt receptor complex [Kawano and Kypta, 2003]. Shifted (Shf), the counterpart of WIF1, appears to function as a positive component of hedgehog signaling in Drosophila [Wang et al., 2007].
Both hedgehog and Wnt signaling are involved in tumors of various types [Taipale and Beachy, 2001; Cohen, 2003a,b]. Known mutations in hedgehog signaling are summarized in Table I and in Wnt signaling in Table II.
| Condition | Mutated genes |
|---|---|
| Colorectal adenomas | APC |
| CTNNB1 | |
| Colorectal adenocarcinomas (includes Gardner syndrome variant) | APC |
| CTNNB1 | |
| Desmoid tumors | APC |
| Hepatoblastoma | APC |
| CTNNB1 | |
| Adrenocortical adenoma | APC |
| Adrenocortical carcinoma | APC |
| Uterine adenocarcinoma | APC |
| Papillary thyroid carcinoma | APC |
| Medulloblastoma | APC |
| CTNNB1 | |
| Endometrial ovarian carcinoma | CTNNB1 |
| Pilomatricomas | CTNNB1 |
| Colorectal cancer | AXIN2 |
| Oligodontia-colorectal cancer syndrome | AXIN2 |
- Updated from Cohen [2003b].
Evolutionary origin of Wnt and Hh has been addressed by Taipale and Beachy 2001 and Nusse 2003. C. elegans contains five different Wnt genes and three β-catenins. The hydra contains a Wnt gene as well as a set of Wnt pathway genes. No hedgehog-like genes have been found in either C. elegans or in the hydra, but the structure of Hh is similar to other enzymes, particularly bacterial ones, suggesting that Hh may be derived from an ancient metabolic pathway.
PRIMARY CILIUM IN VERTEBRATE HEDGEHOG SIGNALING
Cilia are present in most eukaryotic cells except for those for fungi and higher plants. They are ubiquitous in vertebrate cells with the exception of bone marrow cells and the intercalated cells of the kidney collecting duct [Praetorius and Spring, 2005].
Signals from primary cilia are ultimately involved in regulating the cell cycle, cytoskeletal organization, intraflagellar transport, and various signaling pathways, such as those of hedgehog and Wnt. The cilium is a microtubule-based axoneme projecting from the cell surface into the extracellular space (Fig. 8A). The axoneme itself consists of nine peripheral double tubules arranged around a core containing either two central microtubules for motility or no central microtubules for sensory ceils [Bisgrove and Yost, 2006] (Fig. 8B).
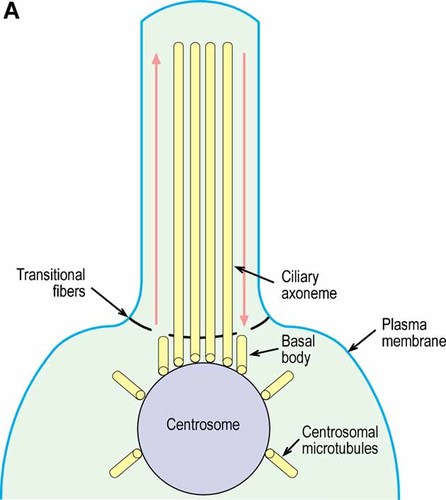
The cilium is a microtubule-based axoneme consisting of nine peripheral double tubules derived from two inner microtubules of the basal body (this two dimensional view permits only four of the axoneme microtubules to be shown). Interflagellar transport is represented by red up and down arrows. Based on data from Bisgrove and Yost 2006.
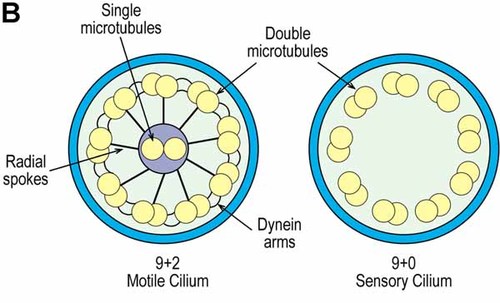
Cross section of a cilium. The nine double microtubules of the axoneme are arranged around a core containing either two central microtubules for motility (9+2) or no central microtubules for sensory cells (9+0). Based on data from Bisgrove and Yost 2006.
The primary cilium only assembles when vertebrate cells are not in mitosis. Just preceding cell division, the cilium is reabsorbed. Following cell division, ciliogenesis reconstitutes the microtubule-based axoneme of the primary cilium from the basal body [D'Angelo and Franco, 2009; Simpson et al., 2009].
Non motile (sensory) cells of the 9+ 0 type (Fig. 8B) are generally considered to be chemical or mechanical sensors and are known as primary cilia. Motile cilia of the 9 + 2 type (Fig. 8B) tend to have multiple cilia, as in respiratory epithelial cells responsible for mucus clearance or in ependymal cells responsible for ependymal flow [D'Angelo and Franco, 2009; Simpson et al., 2009].
The ciliary tip harbors the microtubule ends of the axoneme, (1) which grow and (2) which is the point where switching occurs from anterograde (up arrow in Fig. 8A) to retrograde (down arrow in Fig. 8A) intraflagellar transport (IFT). Movement of protein particles up the axoneme involves the heterotrimeric motor kinesin-2 and retrograde trafficking is mediated by a dynein motor [Fliegauf et al., 2007; Simpson et al., 2009].
Through a transition zone between the cilium and the cell, the microtubular structure of the basal body forms the nine peripheral tubules of the cilium (Fig. 8A) [Fliegauf et al., 2007]. Seventeen IFT proteins have been identified and make up a large complex that transports cargo along the axonemal tubules of the cilium [Rosenbaum and Witman, 2002; Bisgrove and Yost, 2006]. Three IFTs are required for hedgehog signaling at some point between Smo and Gli transcription factors [Huangfu et al., 2003; Huangfu and Anderson, 2005; Liu et al., 2005].
In the absence of hedgehog ligand, Ptch1 localizes to the primary cilium, where it inhibits Smo (Figs. 9A, left and 9B, left). When hedgehog binds to Ptch1, the inhibition is released and Smo shuttles from an endocytic vesicle to the cilium (Fig. 1), while the hedgehog-patched complex enters an endocytic vesicle (Figs. 9A, right and 9B, right).
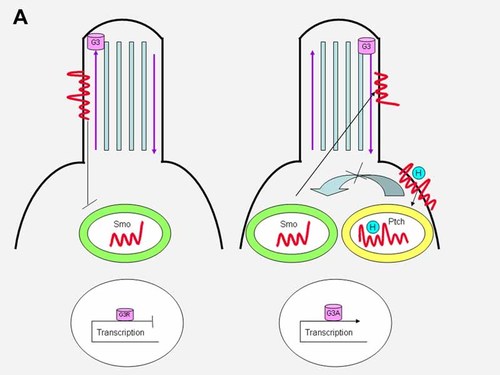
In the absence of hedgehog ligand, Ptch1 localizes to the primary cilium, where it inhibits Smo (left). When hedgehog binds to Ptch1, the inhibition is released, Smo shuttles from an endocytic vesicle to the cilium, and the hedgehog–patched complex enters an endocytic vesicle (right). Gli3 (G3) has both activator (G3A) and repressor (G3R) forms. See text.
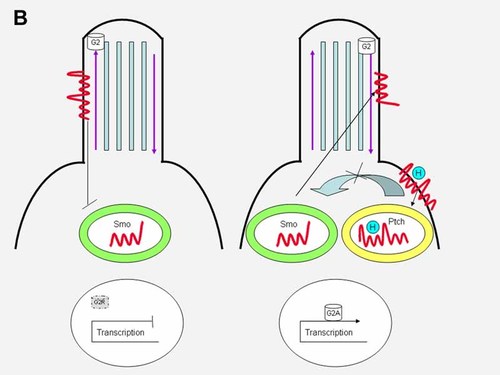
In the absence of hedgehog ligand, Ptch1 localizes to the primary cilium, where it inhibits Smo (left). When hedgehog binds to Ptch1, the inhibition is released, Smo shuttles from an endocytic vesicle to the cilium, and the hedgehog–patched complex enters an endocytic vesicle (right). Gli2 (G2) has only an activator (G2A) form, but no repressor form because it is degraded (see gray G2R with dotted outline, indicating degradation). See text.
The main targets of hedgehog signaling are the Gli transcription factors. Gli3 has both activator and a repressor forms. In the absence of hedgehog binding to Ptch1, Gli3 located on the anterograde tip of the cilium is cleaved into its transcriptional repressor form through limited proteasome-mediated degradation and blocks transmission of downstream target genes (Fig. 9A, left). This involves a C-terminal processing determinant domain (PDD) on Gli3 [Pan and Wang, 2007]. In the presence of hedgehog binding to Ptch1, Gli3 as a full length transcriptional activator switches from the anterograde tip of the cilium to its retrograde side and activates downstream target genes (Fig. 9A, right). Gli2 has only an activator form in the presence of hedgehog ligand binding with a similar switch of full length Gli2 from the anterograde ciliary tip to the retrograde side and activation of downstream target genes (Fig. 9B, right). Gli2 has no repressor form through proteasome-mediated degradation (Fig. 9B, left) [Pan and Wang, 2007]. Like Gli2, Gli1 has only an activator form. In conclusion, the processing of Gli3 and the degradation of Gli1 and Gli2 share similar mechanisms, but result in different outcomes [Wang et al., 2007].
The function of cilia is critical for proper left/right asymmetry and primary ciliary dyskinesia in which situs is randomized, as in heterotaxy from immobility of Nodal cilia [Southerland and Ware, 2009] (see section titled “Nodal Signaling Pathway”).
The primary cilium also plays a role in the Wnt signaling pathway by acting as a switch between canonical Wnt/β-catenin signaling and the non-canonical Wnt/planar cell polarity pathway [Bisgrove and Yost, 2006; Fliegauf et al., 2007; He, 2008].
HEDGEHOG SIGNALING IN VERTEBRATES
Bilipidated hedgehog becomes processed as lipoprotein-associated multimers by Disp, exiting the signaling cell and traveling via glypicans and megalin to the responding cell. Megalin regulates hedgehog protein turnover. For simplicity in the diagram (Fig. 6), hedgehog travels from the signaling cell to the responding cell, whereas in actuality, it travels across many cell diameters. In the responding cell, hedgehog interacts with a complex of Ptch1 and Cdo/Boc, which activates endocytic Smo to the cilium. Ptch1 regulates the activity of Smo (1) via vitamin D3, which inhibits Smo in the absence of hedgehog ligand or (2) via oxysterols, which activate Smo in the presence of hedgehog ligand. Hedgehog ligands also interact with Hip1, Ptch2, and Gas1, which regulate the range as well as the level of hedgehog signaling [Huangfu and Anderson, 2006; Wang et al., 2007].
Cell Surface and Extracellular Matrix Proteins
Several cell surface and extracellular matrix proteins have been identified and these have added increased complexity to hedgehog signaling. Cdo and Boc in vertebrates and their counterparts ihog and boi in Drosophila help regulate hedgehog signaling in a positive manner by binding to the hedgehog protein [Tenzen et al., 2006; Yao et al., 2006; Zhang et al., 2006a]. This enhances signaling in a synergistic interaction with Ptch1 [Hooper and Scott, 2005].
Shifted (Shf) is also found in both Drosophila and in the vertebrates. Shf is the counterpart of WIF1. However, unlike WIF1, which acts as an antagonist in Wnt signaling (see Similarities of Hedgehog and Wnt Signaling Section), Shf appears to function in a positive manner by regulating the extracellular distribution of hedgehog ligand [Glise et al., 2005; Gorfinkiel et al., 2005].
Mammalian Scube2 and its zebrafish ortholog you are extracellular regulators of hedgehog signaling [Kawakami et al., 2005; Woods and Talbot, 2005; Hollway et al., 2006]. Although it is not yet clear how Scube2 and you function, they act upstream of Smoothened and may possibly regulate the distribution of hedgehog ligand, or alternatively, perhaps permit hedgehog signaling indirectly by antagonizing the BMP pathway [Wang et al., 2007].
Tectonic is required to activate hedgehog signaling in the mouse and apparently acts downstream of Smoothened [Reiter and Skames, 2006].
Hedgehog Interacting Protein 1 (Hip1) and Growth Arrest Specific 1 (Gas1)
Hip1, found in vertebrates but not in Drosophila, encodes a membrane-bound glycoprotein that binds Shh with attenuates hedgehog signaling, thus reducing its effective range [Chuang and McMahon, 1999]. Gas1 is a 45 kDa glycophosphatydlinosotol (GPI)-linked protein originally for arresting the cell cycle when overexpressed [Del Sal et al., 1992]. It has been suggested that Gas1 attenuates hedgehog signaling, thus reducing its effective range [Lee et al., 2001]. Kang et al. 2007 found in “cooking with Gas1” that Gas1 is associated with Cdo in patterning the neural tube and the midface. In Figure 6, Ptch2 interacts with both Hip1 and Gas1 and together with ligand binding regulates the range and level of hedgehog signaling [Wang et al., 2007].
Intracellular Transduction
Costal 2 (Cos2), a microtubule-associated kinesin-like motor protein, so important in Drosophila transduction [Kamal and Goldstein, 2002; Mandelkow and Mandelkow, 2002] plays no role in mammalian transduction. In fact, the putative mammalian homologs of Cos2, Kif7, and Kif27, do not play a significant role in mammalian transduction [Wang et al., 2007]. Evidence is accumulating to suggest that the cilium may provide a function analogous to that of Cos2 by linking Smo to the modulation of Gli transcription factors [Corbit et al., 2005; Haycraft et al., 2005; Huangfu and Anderson, 2005]. Suppressor of fused (Sufu) has acquired an essential function in mammals. Fused (Fu), a serine–threonine kinase, so essential in regulating hedgehog signaling in Drosophila, plays no obvious role in the mouse [Chen et al., 2005; Merchant et al., 2005], but may be required in other vertebrates such as in zebrafish. The relative roles of Gli orthologs also differ between the zebrafish and the mouse [Karlstrom et al., 2003]. Although differences between Drosophila and the mouse are not uncommon in signal transduction, the striking differences in regulation in Drosophila, zebrafish, and the mouse are not typical of other pathways and, in fact, may be related to the presence or absence of acquisition or loss of cilium-based mechanisms of pathway regulation [Wang et al., 2007].
Rab23
The term Rab stands for Ras-like in rat brain. Rab proteins are small GTPases of the Ras superfamily that regulate intracellular membrane trafficking. A null mutation open brain (opb−/−) results in an open neural tube involving the head and spinal cord, abnormal somites, polydactyly, and poorly developed eyes [Günther et al., 1994]. The opb mutation was found to arise from the Rab23 gene [Eggenschwiler et al., 2001]. Rab23 is a negative regulator of hedgehog signaling [Evans et al., 2003]. Rab23 acts downstream of Smo but upstream of Gli2, Gli3, and the IFT proteins [Huangfu and Anderson, 2006]. The human RAB23 gene, which maps to 6p11, encodes a 237 amino acid protein. Jenkins et al. 2007 showed that RAB23 mutations caused Carpenter syndrome and because of the open neural tube defect in Rab23 null mice Jenkins et al. 2007 suggested a species difference in the requirement for Rab23 during early development. Other known human RAB mutations include autosomal dominant Charcot–Marie–Tooth disease type 2B (RAB7 mutations) and autosomal recessive Griscelli syndrome type 2 (RAB27A mutations) [Ménasché et al., 2000; Verhoeven et al., 2003].
Gli Gene Family
GLI1 was identified originally as an amplified gene in a human glioblastoma [Kinzler et al., 1987, 1988; Kinzler and Vogelstein, 1990]. GLI1, GL2, and GLI3 (Table III) encode transcription factors that share five highly conserved tandem C2H2 zinc fingers and a conservative histidine–cysteine linker sequence between zinc fingers [Rupert et al., 1988]. The three GLI genes vary in size: GLI3 > GLI2 > GLI1. GLI1 has two isoforms; GLI2 has three alternatively spliced exons; and GLI3 has only one isoform. GLI1, encoding a 1,106 amino acid protein, maps to 12q13; GLI2, encoding 810, 829, 1241, or 1,258 amino acids, maps to 2q14; GLI3, encoding 1,595 amino acids, maps to 7p13 [Rupert et al., 1988, 1990; Matsumoto et al., 1996; Reifenberger et al., 1996; Biesecker, 2004]. The functions and phenotypic effects of GLI1, GLI2, and GLI3 are summarized in Table III. GLI3 has both activator and a repressor forms; the activator form utilizes cAMP response element-binding protein (CBP) as a co-activator [Partanen, 2001].
| GLI genes | Chromosome map locations | Forms | Functions | Phenotypic effects |
|---|---|---|---|---|
| GLI | 12q13 | Two isoforms | Activator form only | GLI1↑ with basal cell carcinoma |
| GLI2 | 2q14 | Three alternatively spliced exons | Activator form only | Mutations for holoprosencephaly |
| GLI3 | 7p13 | One isoform | Activator and repressor forms | Mutations for Pallister–Hall syndrome, Greig cephalopolysyndactyly, and medulloblastoma |
- See text, particularly for the processing of GLI3 and the degradation of GL1 and GLI2.
Unfortunately, Human Krüppel-Related Gene 4 (HKR4), which maps to 8q24.3, has been misclassified as a member of the GLI gene family and was called GLI4 by Kas et al. 1996. Also, in Drosophila, the term Gli refers not to the Gli gene family, but to the Drosophila gene Gliotactin [Rupert et al., 1988].
Recent studies show that the molecular mechanism of Gli3 processing requires the same set of kinases required for Ci in Drosophila: PKA, CKI, and GSK3β [Jia et al., 2004; Zhang et al., 2006b; Tempe et al., 2006; Wang and Li, 2006]. In vertebrates, PKA begins the sequential phosphorylations of Gli3 and leads to the binding of βTrCP, the vertebrate homolog of Slimb, and the subsequent processing of Gli3 [Tempe et al., 2006; Wang and Li, 2006]. In contrast, both Gli1 and Gli2 are degraded instead of being processed. The degradation of Gli2 is inhibited by hedgehog signaling [Pan et al., 2006]. The same kinases that act on Gli3 also phosphorylate Gli2, leading to Gli2 association with βTrCP and ubiquitin proteasome-mediated degradation. Gli1 undergoes PKA- and βTrCP-dependent degradation [HuntZicker et al., 2006]. Thus, the processing of Gli3 and the degradation of Gli1 and Gli2 share similar mechanisms, but result in different outcomes [Wang et al., 2007].
Several other regulator factors are involved in processing Ci and Gli. HIB, a hedgehog-induced protein that contains MATH and BTB domains, is a negative regulator of hedgehog signaling in Drosophila that promotes ubiquitination and degradation of Ci. SPOP, the mammalian homolog of HIB, is involved in Gli degradation [Zhang et al., 2006b]. In contrast, PI3-kinase-dependent activation of Akt regulates Shh signaling by controlling phosphorylation of Gil2 [Riobo et al., 2006]. In the chicken, the Talpid3 gene is essential for Gli3 processing and Gli activator functions [Davey et al., 2006].
Cyclic AMP Response Element-Binding Protein (CBP)
The cAMP-regulated enhancer (CRE) binds many transcription factors including CRE-binding protein (CRB), which is activated as a result of phosphorylation by protein kinase A (PKA) at a single amino acid residue, serine133. CBP is a 2,441 amino acid 265 kDa nuclear protein, which is a co-activator for a number of transcription factors including GLI3. CBP maps to 16p13.3 [Chrivia et al., 1993; Petrij et al., 1995].
Target Genes
Hedgehog signaling affects almost all portions of the vertebrate body plan during development. This is made possible by the various cellular responses to Hedgehog, including (1) the type of responding cell, (2) the dose of Hedgehog received, and (3) the timing during which the cell is exposed [Varjosalo and Taipale, 2008].
Target genes (1) can be positive or negative [Varjosalo and Taipale, 2008], (2) may be identified by genome-wide expression profiling [McGlinn et al., 2002; Xu et al., 2006], and (3) are being discovered on an ongoing basis [Varjosalo and Taipale, 2008].
Target genes include, among others, BMP4 [Astorga and Carlsson, 2007], FGF4 [Laufer et al., 1994], VEGF [Pola et al., 2001], Myf5 [Gustafsson et al., 2002], Ptch1, Ptch2, Nkx2.2, Nkx2.1, Rab34 [Vokes et al., 2007], Pax6, Pax7 [Wang et al., 2007], Pax9, Jagged1 [McGlinn et al., 2002], genes involved in cell growth and division (e.g., N-Myc) [Oliver et al., 2003], and many transcription factors (other members of the Myod/Myf, Pax, Nkx, Dbx, and Irx families) [Pierani et al., 1999; Gustafsson et al., 2002; Jacob and Briscoe, 2003; Vokes et al., 2007; Varjosalo and Taipale, 2008].
GENETIC DISORDERS ASSOCIATED WITH THE HEDGEHOG SIGNALING NETWORK
Tables of Mutations
The following sections contain both text and tables of mutations for disorders of the hedgehog signaling network. In recent years, recommendations for molecular mutations have changed a number of times so that yesterday's nomenclature is replaced with today's nomenclature and it is predictable that there will be further recommendations for tomorrow's nomenclature. This being the case, I have elected to maintain the original nomenclature from each table to make it more user-friendly for readers who wish to review the original sources.
Nevoid Basal Cell Carcinoma Syndrome and PTCH1 Mutations
The nevoid basal cell carcinoma syndrome (Fig. 10) is an autosomal dominant disorder characterized by skin manifestations (multiple basal cell carcinomas in about 90%, epidermal cysts, and palmar and plantar pits), skeletal defects (bifid ribs, cervical spina bifida occulta, kyphoscoliosis, pectus excavatum, Marfanoid habitus in some cases, short fourth metacarpals, and postaxial polydactyly in about 1–4%), CNS abnormalities (medulloblastoma in about 3–5%, calcification of the falx cerebri, bridging of the sella turcica, and meningioma in about 1% or less), craniofacial features (macrocephaly, mild hypertelorism, well developed supraorbital ridges resulting in an appearance of sunken eyes, relative mandibular prognathism, and cleft lip/palate in about 5%), oral manifestations (odontogenic keratocysts of the jaws in about 90%), and other findings (lymphomesenteric cysts, ovarian fibromas, often bilateral in about 15%, and cardiac fibroma in about 3%) [Gorlin, 1987; Cohen, 1999].
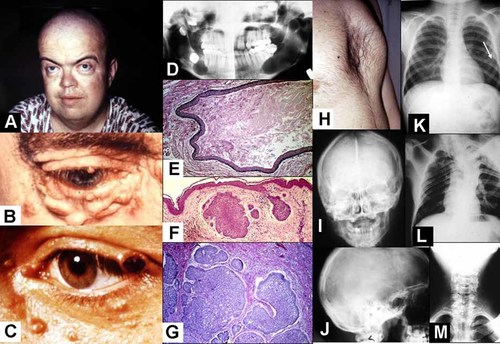
Nevoid basal cell carcinoma syndrome. A: Macrocephaly, mild hypertelorism, and prominent supraobital rims, giving the appearance of sunken eyes. B: Basal cell carcinoma nodules. C: Ulceration of a basal cell carcinoma on the eyelid margin. D: Panographic radiograph showing jaw cysts. E: Histological appearance of an odontogenic keratocyst. Note stratified squamous epithelial lining and the sloughed off keratin within the cyst. F: Histological appearance of two basal cell carcinomas. G: Histological appearance of several basal cell carcinomas. Note peripheral palisading of the outer epithelial cells. H: Pectus excavatum. I: Calcification of the falx cerebri. J: Bridging of the sella turcica. K: Note bifid ribs (arrow). L: Kyphoscoliosis. M: Cervical spina bifida occulta.
The syndrome is caused by mutations in PTCH1, the human homolog of the Drosophila segment polarity gene Ptch1. PTCH1 is a tumor suppressor gene located at 9q22.3. It functions as a cell cycle regulator, stopping cell division in the absence of SHH ligand and permitting cell division when ligand binding occurs [Cohen, 1999].
Generally, for a tumor suppressor gene to be inactivated, two hits are required. The first hit involves a mutation in one allele which can be dominantly inherited if present in a germ cell. The second hit involves the other allele, resulting in loss of heterozygosity by deletion, mitotic nondisjunction, or mitotic recombination. PTCH1 is probably not a classic tumor suppressor gene because the first hit causes major features such as a Marfanoid habitus, macrocephaly, relative mandibular prognathism, spina bifica occulta, postaxial polydactyly, and cleft lip/palate. Loss of heterozygosity has been demonstrated in basal cell carcinomas, odontogenic keratocysts, and medulloblastomas [Cohen, 1999].
PTCH1 germline mutations for the nevoid basal cell carcinoma syndrome are summarized in Table IV and mutations for sporadic basal cell carcinomas are summarized in Table V. Germline mutations (see Fig. 11) are mainly of the truncating type and they occur most commonly in the large extracellular loops, the large intracellular loop, and the N-terminal region. Germline missense mutations occur predominantly in the transmembrane domains, particularly transmembrane 4, which is located in the SSD. Missense mutations in sporadic basal cell carcinomas are clustered in the first large extracellular loop, the large intracellular loop, and in the C-terminal region [Lindström et al., 2006]. No correlations have been observed between the position of germline mutations and the phenotype of the nevoid basal cell carcinoma syndrome [Wicking et al., 1997; Lindström et al., 2006]. Some instances of PTCH1 mutations for the nevoid basal cell carcinoma syndrome represent examples of somatic mosaicism [Wicking et al., 1997].
| Type of mutation | Exon | Nucleotide change |
|---|---|---|
| 2 bp deletion | 2 | 244delCT |
| 23 bp deletion | 2 | del253-375 |
| 1 bp insertion | 2 | 269insT |
| Deletion, substitution | 2 | 277AA → C |
| Premature stop | 3 | 391C → T |
| 2 bp insertion | 3 | 464insAC |
| 1 bp insertion | 5 | 693insC |
| 37 bp deletion | 6 | del804-840 |
| 1 bp insertion | 6 | 853insC |
| 1 bp deletion | 6 | 929delC |
| Missense | 8 | 1067T → G (Leu360Arg) |
| Premature stop | 8 | 1081C → T |
| Premature stop | 8 | 1081CC → TT |
| Premature stop | 8 | 1148G → A |
| Premature stop | 10 | 1368G → A |
| 76 bp deletion | 10 | del370-1445 |
| 8 bp duplication | 11 | dup1497-1504 |
| Missense | 11 | 1513G → A (Gly509Arg) |
| Missense | 11 | 1514G → T (Gly509Val) |
| Missense | 11 | 1525G → T (Asp513Tyr) |
| 1 bp insertion | 13 | 2000insC |
| 2 bp insertion | 13 | 2047insCT |
| Premature stop | 13 | 2050C → T |
| Premature stop | 13 | 2068C → T |
| 2 bp deletion | 13 | 2183delTC |
| 1 bp insertion | 14 | 2320insA |
| 1 bp deleton | 14 | 2392delA |
| 3 bp deletion | 14 | del2434-2436 |
| 11 bp deletion | 15 | del2442-2452 |
| 9 bp insertion | 15 | 2445insCCGAATATC |
| 4 bp deletion | 15 | del2574-2577 |
| 1 bp deletion | 15 | 2583delC |
| Complex ins/del | 16 | 2596complex |
| 1 bp insertion | 16 | 2748insC |
| 7 bp duplication | 16 | dup2749-2755 |
| 5 bp insertion | 16 | 22788ins5 |
| 8 bp deletion | 17 | del2988-2995 |
| 1 bp insertion | 17 | 3014insA |
| Premature stop | 17 | 3015C → A |
| Missense | 18 | 3193G → C (Gly1069Arg) |
| 6 bp deletion | 18 | del3232-3237 |
| 2 bp deletion | 19 | 3352delAT |
| Missense | 19 | 3383C → A (Ser1132Tyr) |
| 1 bp deletion | 21 | 3538delG |
| Missense | 22 | 4302G → T (Glu1438Asp) |
| Type of mutation | Intron | Nucleotide change |
|---|---|---|
| Splicing | 7 | A1055-2C |
| Splicing | 10 | 1493-8ins21 |
| Splicing | 16 | 2875 + G → C |
- Data summarized by Cohen 1999.
| Gene | Type of mutation | Exon | Nucleotide change |
|---|---|---|---|
| PTCH1 | 1 bp insertion | 2 | 278insT |
| Premature stop | 3 | 429TG → AT | |
| Missense | 3 | 451C → T (Pro155Ser) | |
| Missence | 3 | 523C → T (Leu175Phe) | |
| Missense | 3 | 572G → A (Arg195Lys) | |
| 300 bp duplication | 6 | Duplication of exon 6 | |
| Premature stop | 6 | 756G → A | |
| Frameshift | 6 | 845A → G, del846-849 | |
| 2 bp insertion | 7 | 1051ins2 | |
| Premature stop | 8 | 1080CC → TT | |
| Premature stop | 8 | 1081C → T | |
| Premature stop | 8 | 1149G → A | |
| Premature stop | 9 | 1237C → T | |
| 1 bp insertion | 10 | 1382insC | |
| 4 bp deletion | 10 | del422-1425 | |
| Missense | 13 | 1981C → G (Arg665Gly) | |
| Premature stop | 15 | 2554C → T, del2555-2556 | |
| 14 bp deletion | 16 | del2704-2717 | |
| Frameshift | 16 | 2779C → T, del2880-2881 | |
| 1 bp deletion | 16 | 2861delA | |
| Premature stop | 17 | 2953G → T | |
| Premature stop | 17 | 3042G → A | |
| Missense | 21 | 3571A → T (Thr1195Ser) | |
| Missense | 21 | 3574C → T (Pro1196Ser) | |
| Missense | 21 | 3622G → A (Gly1212Ser) | |
| 12 bp deletion | 22 | del3844-3855 | |
| Missense | 22 | 3932T → C (Leu1311Pro) | |
| SHH | Missense (activating) | 2 | 397C → T (His133Tyr) |
| SMO | Missense (activating) | 4 | 595C → T (Arg199Trp) |
| Missense (activating) | 9 | 1604G → T (Trp535Leu) | |
| Missense (activating) | 10 | 1685G → A (Arg562Glu) |
| Gene | Type of mutation | Intron | Nucleotide change |
|---|---|---|---|
| PTCH1 | Splicing | 11 | 1493-8T → C |
| 3′ consensus splite site | 14 | CAG → CAA | |
| 3′ consensus splice site | 17 | CAGG → CAAA |
- Data summarized by Cohen 1999.
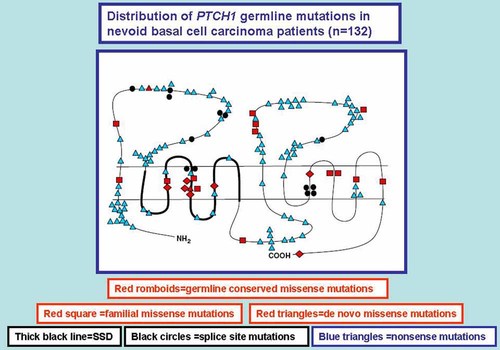
Distribution of germline PTCH1 mutations in nevoid basal cell carcinoma syndrome patients (n = 132) and their placement on the PTCH1 protein. The various types of mutations appear in boxes in the figure. Modified and adapted after Lindström et al. 2006.
A deletion (9q22.1–q22.32) has been noted to include PTCH1 and ROR2 (found in autosomal recessive Robinow syndrome); the authors speculated that pulmonary valve stenosis found in their patient with nevoid basal cell carcinoma syndrome might result from haploinsufficiency of ROR2 [Nowakowska et al., 2007]. Happle 2000 suggested that the few known instances of unilaterally located multiple basal cell carcinomas in the absence of other abnormalities suggests the possibility of a nonsyndromic type of hereditary multiple basal cell carcinomas. One PTCH2 mutation has been reported in a family with nevoid basal cell carcinoma syndrome [Fan et al., 2008] and rare instances of PTCH2 mutations have been noted in instances of isolated basal cell carcinoma and medulloblastoma [Smyth et al., 1999].
DISP1 Mutations
The discussion of DISP1 mutations follows the discussion of PTCH1 and PTCH2 because all three are 12-pass transmembrane proteins with a SSD (see Fig. 3). DISP1, originally known as DISPA based on its murine ortholog DispA, maps to 1q42 [Ma et al., 2002]. Roessler et al. 2009b reported two families with truncating mutations in DISP1 (Table VI). Shaffer et al. 2007 used array-based comparative genomic hybridization identified a microdeletion of 1q41q42. Overlap with DISP1 resulted in features suggestive of microforms of holoprosencephaly, such as developmental delay, seizures craniofacial dysmorphism, microcephaly, cleft palate, and short stature, but no frank holoprosencephaly. The phenotypic features in the two families reported by Roessler et al. 2009b with DISP1 mutations were similar to those found by Shaffer et al. 2007; they suggested that a model of holoprosencephaly based on multiple genetic and/or environmental insults—the multiple hit hypothesis [Ming and Muenke, 2002] may provide the best framework for a more comprehensive understanding of the complex interplay of various predisposing factors in holoprosencephaly.
| Coding position | Comment |
|---|---|
| His159Tyr | Missense change of unknown significance |
| Leu469Ser | Missense change of unknown significance |
| Trp475X | Loss-of-function |
| Gln674His | Missense change of unknown significance |
| Tyr734X | Loss-of-function |
| Phe816Val | Missense change of unknown significance |
| Gln928Arg | Missence change of unknown significance |
Holoprosencephaly
Holoprosencephalic phenotypes are illustrated in Figure 12. Table VII summarizes the variability in their facial features and their CNS anomalies. All genes whose mutations may result in holoprosencephaly are summarized in Table VIII; they are characterized under the followings headings: gene symbols, their meanings, their map locations, and their functions. Table IX summarizes the mutated genes, the variability in their holoprosencephalic phenotypes, and their frequencies.
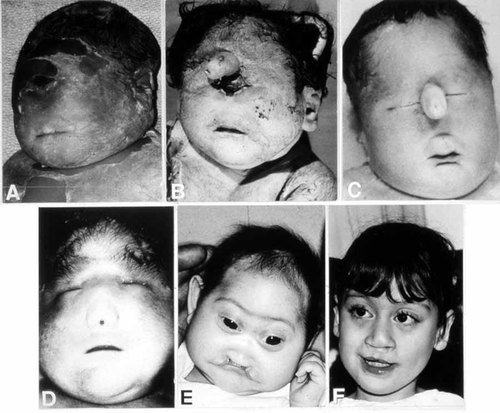
The faces of holoprosencephaly. Top, left: Cyclopia without probosicis. Top, center: Cyclopia with proboscis. Top, right: Ethmocephaly. Bottom, left: Cebocephaly. Bottom, center: Microcephaly, hypotelorism, flat nose, and median cleft lip. Bottom, right: Hypotelorism and repaired cleft lip. From Cohen [1989a].
| Facial typeb | Main facial features | Brain |
|---|---|---|
| Cyclopia | Median monophthalmia, synophthalmia, or anophthalia; proboscis may be single, absent, or rarely double; hypognathia in some cases | Alobar holoprosencephaly |
| Ethmocephaly | Ocular hypotelorism with proboscis | Alobar holoprosencephaly |
| Cebocephaly | Ocular hypotelorism and blind-ended single-nostril nose | Usually alobar holoprosencephaly |
| Median cleft lip | Ocular hypotelorism, flat nose, and median cleft lip | Usually alobar holoprosencephaly |
| Less severe facial dysmorphism | Variable features including ocular hypotelorism or hypertelorism; flat nose, unilateral or bilateral cleft lip, iris coloboma, or other anomalies; minimal facial dysmorphism or even an essentially normal face in some instances | Semilobar or lobar holoprosencephaly |
- a For review see Cohen 2006.
- b Transitional facial forms are known to occur.
| Gene symbol | Meaning of gene symbol | Map location | Function |
|---|---|---|---|
| Hedgehog signaling network | |||
| SHH | Sonic Hedgehog | 7q36 | Ligand for Patched; SHH expression is in the ventral neural tube |
| PTCH1 | Patched 1 | 9q22.3 | Suppresses Smoothened in the absence of Sonic Hedgehog ligand |
| DISP1 | Dispatched 1 | 1q42 | Permits bilipidated Sonic Hedgehog to exit the plasma membrane of the signaling cell |
| GLI2 | Glioma 2 | 2q14 | Activator transcription factor in hedgehog signaling |
| Cholesterol biosynthesis | |||
| DHCR7 | Dehydrocholesterol reductase 7 | 11q12-q13 | Converts 7-Dehydrocholesterol to Cholesterol |
| TGFβ superfamily | |||
| NODAL | Nodal | 10q22.1 | ActR2A/B and ActR1B ligand involved with midline establishment and left/right asymmetry |
| TGIF | TGFβ-lnduced Factor | 18p11.3 | Atypical homeodomain protein that acts as a transcriptional repressor of retinoids and TGFβ |
| FOXH1b | Forkhead Box 1 | 8q24.3 | Transcriptional factor in the Nodal signaling pathway |
| EGF-CFC gene familyc | |||
| TDGF1b | Teratocarcinoma-Derived Growth Factor 1 | 3p21p23 | Co-receptor for Nodal |
| ZIC gene family | |||
| ZIC2 | Zinc Finger Protein of Cerebellum 2 | 13q32 | Axis formation and ZIC2 expression in the dorsal neural tube |
| SIX gene family | |||
| SIX3 | Sine Oculis Homeobox 3 | 2p21 | Development of the forebrain and the eye |
- a See text
- b Two genes previously had other names and are mentioned here only for historical interest: TDGF1 (CR1PTO) [Ciccodicola et al., 1989; Dono et al., 1991] and FOXH1 (FASTI; Forkhead Activin Signal Transducer 1) [Zhou et al, 1998].
- c EGF-CFC (Epidermal Growth Factor – Cripto; FRL-1; Cryptic) [Shen and Schier, 2000].
| Mutated genes | Chromosome map locations | Holoprosencephalic phenotypes | Frequencies |
|---|---|---|---|
| SHH | 7q36 | Great variability; mild-to-severe | 17% of familial cases; 3.7% of sporadic cases |
| ZIC2 | 13q32 | Severe brain defect; normal or mildly dysmorphic face | 3–4% |
| SIX3 | 2p21 | Variable | 4.7% of all holoprosencephaly cases |
| TGIF | 18p11.3 | Variable | 1.5% |
| PTCH1 | 9q22.3 | Variable | Rare (common in nevoid basal cell carcinoma syndrome and in some isolated basal cell carcinomas) |
| GLI2 | 2q14 | Variable | 1.8 |
| DISP1 | 1q42 | Microform of holoprosencephaly: Mild facial features (e.g., cleft lip-palate, hypotelorism, single maxillary central incisor); CNS findings (e.g., seizures, mental deficiency in some cases, no frank holoprosencephaly) | 2 loss-of-function mutations reported |
| NODAL | 10q22.1 | Variable | ∼1% in the NODAL signaling system |
| FOXH1 | 8q24.3 | Variable | As part of the ∼1% in the NODAL signaling system |
| TDGF1 | 3p21-p23 | Variable | Two loss-of-function mutations in 83 familial and 327 sporadic cases of holoprosencephaly |
| DHCR7 | 11q12-q13 | Holoprosencephalic faces | 5–6% of Smith–Lemli–Opitz syndrome cases; 9 reported cases with one instance of cyclopia |
Some Causes of Holoprosencephaly Involving Cholesterol
Table X summarizes some causes of holoprosencephaly involving cholesterol and Figure 13 shows a partial, simplified cholesterol biosynthesis pathway of some of these disorders. Two teratogens—AY9944 and BM15.776 block Δ7-sterol reductase, resulting in the accumulation of 7-dehydrocholesterol and reduced serum cholesterol with the production of holoprosencephaly in rats [Barbu et al., 1984; Kolf-Clauw et al., 1996, 1997; Gofflot et al., 1999]. Triparanol is a teratogen that blocks Δ24-sterol reductase, resulting in the accumulation of desmosterol and reduced serum cholesterol with the production of holoprosencephaly in rats [Roux, 1964]. Lrp2 is the gene responsible for the receptor megalin. An Lrp2−/− null mutation for megalin results in holoprosencephaly in the mouse [McCarthy et al., 2002]. The range plant Veratrum californicum when eaten by pregnant ewes produces holoprosencephaly in newborn lambs; two of the isolated compounds—cyclopamine and jervine—have some similarities to cholesterol (Fig. 14) [Keeler and Binns, 1966; Keeler, 1975]. Both cyclopamine and jervine have also been produced experimentally in other animals [Cohen and Shiota, 2002]. In humans, about 5–6% of Smith–Lemli–Opitz syndrome cases are associated with holoprosencephaly [Kelley et al., 1996].
| Causes | Species | Name of teratogens and their effects |
|---|---|---|
| AY9944 | Rat | Trans-1,4 bis (2-chlorobenzyl-amino-methyl) cyclohexine dihydrochloride. Blocks Δ7-sterol reductase |
| BM15.766 | Rat | 4-(2-[1-(4-chlorocinnamyl) piperazin-4-y]ethyl)-benzoic acid. Blocks Δ7-sterol reductase |
| Triparanol | Rat | MER 29 or 1-[p-β-diethylaminoethoxy)-phenyl]-1-(p-tolyl)-2-(p-chlorophenyl) ethanol. Blocks Δ24-sterol reductase |
| Megalin−/− | Mouse | Megalin is a low-density lipoprotein receptor-related protein (Lrp2). A null mutation (Lrp2−/−) results in holoprosencephaly |
| Cyclopamine | Sheep, rat, mouse, chick, rabbit, hamster | Caused by a steroid isolated from desert plant Veratrum californicum |
| Jervine | Rat, mouse, hamster, treated COS7 cultured cells | Caused by a steroid isolated from desert plant Veratrum californicum |
| Smith-Lemli- | Human | Autosomal recessive malformation syndrome caused by Δ7-sterol reductase mutations |
| Opitz syndrome | About 5–6% of Smith–Lemli–Opitz syndrome cases have holoprosencephaly |
- Table adapted from Cohen and Shiota 2002.
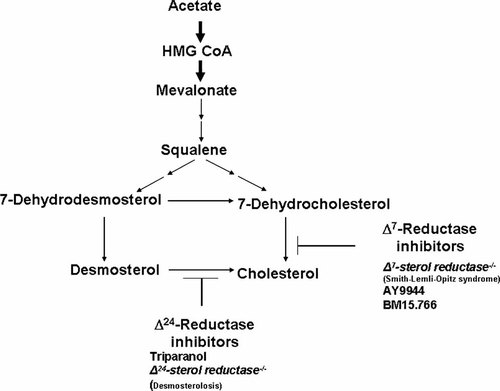
Partial, simplified cholesterol biosynthesis pathway. Double arrows indicate multiple missing steps. Note sterol Δ7-reductase inhibitors: Smith–Lemli–Opitz (SLO) mutations (DHCR7−/−) and teratogens AY9944 and BM15.766. They result in reduced serum cholesterol and an accumulation of 7-dehydrocholesterol. Holoprosencephaly occurs in about 5–6% of Smith–Lemli–Opitz cases. AY9944 and BM15.766 induce holoprosencephaly in rats. BM15.766 and hypomorphic apo B cause holoprosencephaly in mice. Note the sterol Δ24-reductase inhibitors: teratogen triparanol and mutations in human desmosterolosis. Triparanol is associated with various malformations in rats, and holoprosencephaly has been described. The clinical phenotype of human desmosterolosis has not included holoprosencephaly to date, although a hypoplastic corpus callosum has been noted in one case. From Cohen and Shiota 2002.
Impaired Cholesterol Biosynthesis in Human Holoprosencephaly
Of 228 cases of human holoprosencephaly, 9.6% had impaired cholesterol biosynthesis. Subgroups included: (1) one with Smith–Lemli–Opitz syndrome; (2) five with abnormalities of known holoprosencephaly genes (which suggested that defective regulation in cholesterol biosynthesis might add further impairment to hedgehog signaling); and (3) five with an accumulation of cholesterol precursors with variably reduced cholesterol synthesis (which suggested that newly synthesized precursors might have defective transport from the endoplasmic reticulum to the plasma membrane) [Haas et al., 2007; Haas and Muenke, 2010] (see also sections titled “Lipid Adducts Attached to Hedgehog Ligand,” “Studies of the C-Terminal Cholesterol Moiety,” “Sterol Sensing Domain,” and “DHCR7 Mutations and Smith–Lemli–Opitz syndrome”).
Nodal Signaling Pathway
The NODAL signaling pathway plays a central role in the induction of mesoderm and endoderm, and in the specification of left/right asymmetry (Fig. 15, Table VIII). NODAL is a ligand of the TGFβ superfamily. It signals through a heterodimer of activin receptors (ActR1B and ActR2A/B) with Tdgf1 as a co-receptor [Shen, 2007] (Fig. 15). When NODAL binds to the receptor complex, ActR2A/B phosphorylates ActR1B. In turn, ActR1B then phosphorylates Smad2 and Smad3, which interact with Smad4 and activate downstream genes by recruiting FoxHl (Fig. 15).
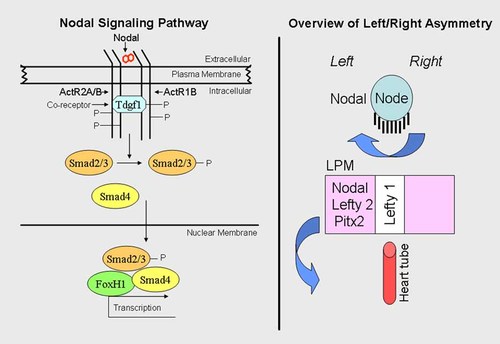
Nodal signaling and left/right asymmetry. Left. Nodal signaling pathway. Nodal is a duplicated ligand that signals through a heterodimer of activin receptors (ActR1B and ActR2A/B) with Tdgf1 as a co-receptor. When Nodal binds to the receptor complex, ActR2A/B phosphorylates ActR1B. In turn, ActR1B then phosphorylates Smad2/3, which interacts with Smad4 and activates downstream genes by recruiting FoxHl. Right. Overview of left/right asymmetry. Prior to the establishment of Nodal cilia, early intracellular asymmetry is evident through planar cell polarity, which also plays a role in the orientation of cilia at the node. Leftward ciliary flow at the node results in asymmetric expression of lateral plate mesoderm (LPM) genes Nodal, Lefty 2, and Pitx2. These then go to form organ primordia (e.g., the heart tube). Right side of diagram based on data from Sutherland and Ware 2009.
NODAL mutations may cause holoprosencephaly, but more commonly result in cardiac anomalies and left/right axis malformations [Roessler et al., 2009c; Solomon et al., 2010] (Fig. 15). FOXH1 mutations can result in cardiac anomalies or in holoprosencephaly [Roessler et al., 2008]. One TDGF1 mutation has resulted in a midline brain malformation and another mutation with frank holoprosencephaly [de la Cruz et al., 2002].
Bone Morphogenetic Protein Signaling Pathway
Bone morphogenetic proteins (BMPs) are members of the TGFβ superfamily. They signal through a heterodimer of BMP receptors (BMPR1 and BMPR2). The binding of BMP ligands to their receptor complexes leads to phosphorylation of BMPR1 by BMPR2. In turn, BMPR1 phosphorylates Smad 1/5/8, and this interacts with Smad4, which then becomes a downstream effector [Geng and Oliver] (Fig. 16).
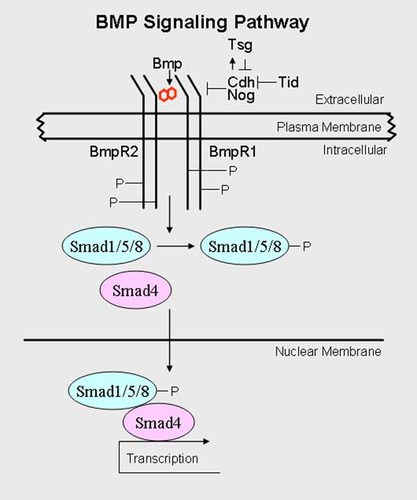
Bone morphogenetic protein signaling. BMP is a double ligand that signals through a heterodimer of BMP receptors (BMPR1 and BMPR2). Binding of BMP ligand to their receptor complexes leads to phosphorylation of BMPR1 by BMPR2. In turn, BMPR1 phosphorylates Smad1/5/8, and this interacts with Smad4, which then becomes a downstream effector, Chd (Chordin) and Nog (Noggin) are secreted antagonists that directly interact with BMP ligands, preventing them from binding to their receptors, Tsg (Twisted gastrulation), also an antagonist, promotes the formation of a stable BMP/Chd/Tsg complex that prevents the binding of BMP ligands to their receptors. See text for the complicated role of Tsg.
Chordin (Chd) and Noggin (Nog) are secreted antagonists that directly interact with BMP ligands, preventing them from binding to their receptors (Fig. 16). Twisted gastrulation (Tsg), an antagonist as well, promotes the formation of a stable BMP/Chd/Tsg complex that prevents the binding of BMP ligands to their receptors. However, the role of Tsg is complicated, and it can also utilize Chd as a substrate for metalloprotease tolloid, which cleaves Chd at specific sites. When Tsg promotes Chd degradation, BMP ligands are then able to signal through their receptors [Geng and Oliver, 2009] (Fig. 16).
Multiple Hit Hypothesis for Holoprosencephaly
Single known genetic mutations for holoprosencephaly in humans account for about 28% of all cases (see Table IX; 10 different genes plus 5–6% of Smith–Lemli–Opitz cases caused by DHCR7 mutations [Dubourg et al., 2007; Kelley and Hennekam, 2000]. A few examples have double heterozygote mutations (see section titled “SHH Mutations”; SHH + ZIC2; 2 examples of SHH + deletion TGIF; GLI2 + PTCH1; and 2 different SIX3 mutations). Double heterozygotes in mice have been discussed by Ming and Muenke 2002.
It is most probable that human cases of holoprosencephaly are multifactorial, being caused by a combination of numerous genetic and environmental factors, and this subject has been discussed by Roessler et al. 2008. These authors found reduced NODAL signaling strength with mutations in several pathway members. Their molecular analysis presages future studies of holoprosencephaly by whole genome analysis.
Multifactorial inheritance includes genetic alterations during embryogenesis, involving for example, the prechordal plate, the position of the anterior portion of the notochord, and early neural tube development [Cohen et al., 1971; Geng and Oliver, 2009]. Although it has been difficult to study how such factors interact in humans [Cohen, 2006; Dubourg et al, 2008; Roessler et al., 2008], studies of a host of genetic and environmental factors in zebrafish, mice, and other animal models have been reviewed by Cohen and Shiota [2002].
In Table XI, mouse models of an alobar holoprosencephaly-like phenotype can result from reduced Nodal signaling, either by (1) introducing a hypomorphic allele (flox) into the Nodal or Cripto null background or (2) by functional inactivation of Gdf1 and genetic deletion of one Nodal allele [Geng and Oliver, 2009].
| Nodal signaling pathway |
| Nodalflox/− |
| Cripto3flox/− |
| Nodal+/− Gdf1−/− |
| Antagonists of the BMP signaling pathway |
| Chd−/− Nog−/− |
| Tsg−/− |
| Nog+/−Chd−/− |
- a Data derived from Geng and Oliver 2009.
Although no mutations in the BMP signaling pathway cause holoprosencephaly, Table XI shows mutations in BMP antagonists that can result in an alobar holoprosencephaly-like phenotype in mice either (1) by digenic inheritance or (2) in Tsg−/− embryos, which can only be observed on a C57BL/6 murine background [Geng and Oliver, 2009].
SHH Mutations
Shh gene expression has been demonstrated in the floor plate of the neural tube. Targeted gene disruption in the mouse produces cyclopia with absence of ventral neural tube cells. The Shh mutation is recessive, resulting in a severe phenotype [Chiang et al., 1996]. In humans, SHH maps to 7q36. In contrast to the mouse, SHH mutations are heterozygous, and the holoprosencephalic phenotype is more variable [Roessler et al., 1996; Nanni et al., 1999].
SHH mutations of the missense, nonsense, deletion, and frameshift types have been identified in exons 1, 2, and 3 (Table XII) [Nanni et al., 1999; Muenke and Beachy, 2001]. Nonsense, deletion, and frameshift mutations may truncate SHH-N, altering biological activity, or interfering with the autoprocessing reaction by affecting SHH-C (Fig. 2). Thus, haploinsufficiency results in holoprosencephaly. Great variability in expression characterizes autosomal dominant SHH families, some being minimally affected with holoprosencephalic microforms and others being severely affected. No genotype/phenotype correlations have been found [Nanni et al., 1999; Muenke and Beachy, 2001; Marini et al., 2003].
| Exon | Nucleotide change | Amino acid change |
|---|---|---|
| 1 | 160-161insGCTG | 3-4ins |
| 189-196del | 13-15del (frameshift) | |
| GCG → AGG | Gly31Arg | |
| GAT → GTT | Asp88Val | |
| CAG → TAG | Gln100Stop | |
| CAG → CAC | Gln100His | |
| 2 | AAG → TAG | Lys105Stop |
| AAC → AAA | Asn → 115Lys | |
| TGG → GGG | Trp117Gly | |
| TGG → CGG | Trp117Arg | |
| TAC → TAG | Tyr158Stop | |
| 3 | GAC → CAG | Glu188Gln |
| CAG → TAG | Gln209Stop | |
| GAC → AAC | Asp222Asn | |
| GTG → GAG | Val224Glu | |
| GCG → ACG | Ala226Thr | |
| AGC → AGA | Ser236Arg | |
| GAG → TAG | Glu256Stop | |
| 939-959del | 263-269del | |
| GAG → TAG | Glu284Stop | |
| GGC → GAC | Gly290Asp | |
| 1283-1291del | 378-380del | |
| GCG → ACG | Ala384Thr | |
| 1361-1375del | 404-408del | |
| CCG → GCG | Pro424Ala | |
| TCG → TTG | Ser436Leu |
- Adapted from Nanni et al. 1999.
Numerous missense mutations occur throughout the entire coding region SHH (Table XII) and their functional significance is more difficult to interpret. If SHH mutations involve haploinsufficiency, holoprosencephaly results, but whether missense mutations alone are sufficient to cause holoprosencephaly has been questioned. Roessler et al. 1996 identified two missense mutations in exon 2, involving an invariant amino acid, Trp117 (Table XII) that immediately follows the crucial α helix-1 motif; they suggested that mutations in Trp117 might destabilize SHH-N. It is also possible that other genes in the sonic hedgehog signaling network or in other developmental pathways might work synergistically with SHH missense mutations. At least three patients have been reported with SHH mutations together with additional gene mutations—one with a ZIC2 mutation and two with deletions involving TGIF [Nanni et al., 1999; Gripp et al., 2000]. There are other double mutations which do not involve SHH: GLI2 and PTCH1 [Rahimov et al., 2006] and double SIX3 mutations [Ribeiro et al., 2006a]. In mice, double heterozygotes result in holoprosencephaly, whereas single heterozygotes do not (Table XIII) [Nomura and Li, 1998; Ming and Muenke, 2002]. Nanni et al. 2001 reported an unusual autosomal dominant family with a single maxillary central incisor and a novel SHH mutation (331A → T, resulting in Ile111Phe), not found in the holoprosencephaly population. El-Jaick et al. 2005 reported a case of alobar holoprosencephaly in a proposita whose mother had only a single maxillary central incisor; an SHH mutation, also involving the 111th amino acid residue, was identified (332T → A, resulting in Ile111Asp).
| Nodal+/− + Smad2+/− |
| Nodal+/− + Hnf3B+/− |
| Nodal+/− + ActRIIA+/− |
Schimmenti et al. 2003 reported a three generation pedigree in which the proband and his mother had iris and uveoretinal colobomas without optic nerve involvement. A novel SHH 24 bp deletion in the 3′ end of the gene was identified.
BMP4 and hedgehog signaling genes (SHH, PTCH1) are expressed co-spatially and co-temporally in the eye, brain, and hand of developing human embryos; Bakrania et al. 2008 identified four cases of anophthalmia–microphthalmia with mutations in both BMP4 and SHH/PTCH1, suggesting synergism between the two pathways in ocular development.
ZIC2 Mutations
ZIC2, which maps to 13q32, is a homolog of the Drosophila odd-paired-like (opa) gene [Aruga et al., 1996] and the zebrafish odd-paired-like (opl) gene. Muenke et al. 2002 suggested that Zic2 is expressed in the dorsal neural tube, in contrast to Shh, which is expressed in the ventral neural tube. It appear that during midgastrulation, Zic2 functions precede those of Shh [Warr et al., 2008].
In humans, heterozygous ZIC2 mutations cause holoprosencephaly. Most patients have alobar or semilobar holoprosencephaly, but lobar holoprosencephaly and syntelencephaly have been noted on occasion [Brown et al., 2001; Muenke et al., 2002]. Before ZIC2 was identified and mapped to 13q32, Cohen 1989a,b observed that in patients with del(13q), facial dysmorphism was mild. Brown et al. 2001 studied 28 patients with ZIC2 mutations and found no instances of cyclopia, ethmocephaly, cebocephaly, or median cleft lip, but rather a normal or mildly dysmorphic face. Solomon et al. 2009b found ZIC2 mutations in 8.5% of probands with holoprosencephaly in large series: 153 individuals from 116 unrelated kindreds, including 137 patients with molecularly determined ZIC2 mutations and 16 patients with whole-gene deletions. A novel and distinct facial phenotype was described and included bitemporal narrowing, upslanting palpebral fissures, short nose with anteverted nares, broad philtrum, and large ears.
ZIC2 mutations have been identified by many authors [Brown et al., 1998, 2001, 2005; Orioli et al., 2001; Dubourg et al., 2004; Roessler et al., 2009a]. In humans, heterozygous ZIC2 mutations cause holoprosencephaly either (1) by small insertions or deletions, leading to frameshift and premature stop codons, thus truncating the protein, or (2) by alanine tract expansion [Brown et al., 1998, 2001; Dubourg et al., 2002]. Brown et al. 2002 observed that many mutations occurred in the carboxy-terminus outside the DNA-binding domain, including some patients with identical expansions of a carboxy-terminal alanine tract. In these instances, DNA-binding is normal, suggesting that the transactivating function of the ZIC2 protein is interrupted, and the protein–protein interactions are critical for normal activity. Muenke et al. 2002 found that 2 of 19 families with ZIC2 mutations had 3 pregnancies resulting in anencephaly. Targeted Zic2 mutations in mice can produce neural tube defects as well as holoprosencephaly [Nagai et al., 2000]. Brown et al. 2002 found no ZIC2 mutations in human neural tube defects, but suggested a possible association with polyhistidine tract polymorphism in the ZIC2 gene. The quintessential study of ZIC2 mutations is that of Roessler et al. 2009a who analyzed 21 previously reported cases and 62 novel mutations (Table XIV). They found loss-of-function to be the predominant mechanism in ZIC2 mutations.
| Base-pair alterations | Coding region alterations |
|---|---|
| −24C > T | Not applicable |
| c.21delG | p.Gln8SerfsX3 |
| c.81_86delGGCGGCinsTCGGT | p.Ala28ArgfsX |
| c107A > C | p.Gln36Pro |
| c.109.A | p.Asp37Pro |
| c.129_184dup56 | p.Leu62Argfs175 |
| c.136C > T | p.Gln46X |
| c.172G > T | p.Gly58X |
| c.177ins56 | p.Phe60GlnfsX |
| c.191dupC | p.Ala66ArgfsX |
| c.217C > T | p.Gln73X |
| c217delC | p.Gln73Argfs1 |
| c.367delA | p.Ser123AlafsX95 |
| c382G > A | p.Asp128Asn |
| c.386_392delCGGCGCC | p.Ser129TrpfsX28 |
| c.392_398del7 | p.Gly133SerfsX83 |
| c.454_455delinsTT | p.Asp152Phe |
| c.479delC | p.Pro160ArgfsX58 |
| c.490G > T | p.Glu164X |
| c.557-572dup6 | p.Gln193AsnfsX180 |
| c.577delC | pGln193AsnfsX25 |
| c.582C > A | p.Tyr194X |
| c.612delC | p.Tyr205ThrfsX13 |
| c.659delA | p.Asn220ThrfsX4 |
| c.665_676dupl2 | p.Gly222_Met225dup |
| c.710_718dupCACCACCAC | p.H12 variant |
| c.716_718del | p.H8 variant |
| c.716_718dupACC | p.H10 variant |
| c.748C > T | p.Gln250X |
| c.779G > A | p.Trp260X |
| c.793C > T | p.Gln265X |
| c.808_809ins17 | p.Lys270ThrfsX2 |
| c.815G > A | p.Ser272Asn |
| c.825_826delA | p.Lys275AsnfsX91 |
| c.829_830dupTT | p.Thr279AlafsX7 |
| c.862_863delTC | p.Ser288GlyfsX78 |
| c.857A > T | p.His286Leu |
| c.858C > G, also c.1059C > T | p.His286Gln, also p.His353His |
| c.856C > T | p.His286Tyr |
| c.871C > T | p.His291Tyr |
| c.910T > A | p.Trp304Arg |
| c.912G > A | p.Trp304X |
| c.928G > T | p.Glu310X |
| c.932delG | p.Gly311AlafsX102 |
| c.941T > G | p.Phe314Cys |
| c.973C > A | p.Arg325Ser |
| c.974G > T | p.Arg325Leu |
| c.979C > T | p.His327Tyr |
| c.1004G > T | p.Cys335Phe |
| c.1025_1026delAA | p.Lys342SerfsX24 |
| c.1031_1032delTC | p.Phe344CysfsX22 |
| c.1040_1046del | p.Glu348erfsX63 |
| c.1051A > T | p.Lys351X |
| c.1052_1053insAAGGTTCACACAGAACCTCAA | p.Lys351_Ile352ins7 |
| c.1075 + 2T > A (IVS1 + 2T > A) | Gly359fsX62 |
| c.1076-1G > A (IVS1-1G > A)a | Alternative splicing |
| c1239 + 1G > C (IVS2 + 1G > C) | Possible inclusion of intron 2 codons or alternative splicing |
| c.1240-2A > G (IVS2-2A > G) | Alternative splicing |
| c.1090C > T | pGln364X |
| 1091_1092delTGb | p.Cys365X |
| c.1097_1098delAG | p.Glu366Valfs2 |
| c.1119_1120delCT | p.Phe374ArgfsX17 |
| c1118G > C | p.Arg373Pro |
| c.1206C > G | p.Tyr402X |
| c.1204T > A | p.Tyr402Asn |
| c.1208C > A | p.Thr403Lys |
| c.1211A > G | p.His404Arg |
| c.1225C > T | p.Arg409Trp |
| c.1245T > G | p.His415Gln |
| c.1277delC | p.Pro426ArgfaX129 |
| c.1313dupC | p.Leu440AlafsX90 |
| c.1323dupG | p.Ser442Valfs88 |
| c.1330_1365del | p.444_455del (in frame deletion) |
| c.1377_1406dup30 | p.Ala461_470dup (in frame duplication) |
| c.1392_1406del | In frame deletion |
| c.1377_1406del | In frame deletion |
| c.[1420-1427dup; 1428-1433delinsCG] | p.Gly477CysfsX54 |
| c.1445_1461del17 | p.Ser482ArgfsX42 |
| c.1452_1456delCGCGG | p.Ala485ArgfsX43 |
| c.1455_1461delCG | pGly487LeufsX41 |
| c.1508_1520delGCGGCGGGGCGG | p.Gly503AlafsX48 |
| c.1559delA | p.His520ProfsX35 |
- Mutations in this table have been adapted from Roessler et al. 2009a (21 are previously published and 62 are novel).
- a Three of the same mutations are shown by Roessler et al. 2009a but only one example appears in this table.
- b Two of the same mutations are shown by Roessler et al. 2009a but only one example appears in this table.
SIX3 Mutations
The so/SIX family of transcription factors consists of a distantly related group of homeobox-containing genes characterized by the presence of a SIX domain (contiguous homology domain), which participates in transcriptional activation [Kawakami et al., 1996]. Six3 is expressed in the developing forebrain and the eyes and in all vertebrates including humans [Oliver et al., 1995; Kobayashi et al., 1998; Loosli et al., 1999; Lacbawan et al., 2009]. Human SIX3 maps to 2p21 [Schell et al., 1996; Wallis et al., 1999]. Geng et al. 2008 and Jeong et al. 2008 showed that Six3 is direct regulator of Shh expression by demonstrating a cross-regulatory loop between Shh and Six3 in the ventral forebrain.
In a cohort of 800 patients with holoprosencephaly, Lacbawan et al. 2009 detected SIX3 mutations in 4.7% of affected probands. SIX3 mutations result in significantly more severe holoprosencephaly than in other cases of nonchromosomal, nonsyndromic holoprosencephaly [Ribeiro et al., 2006a; Lacbawan et al., 2009].
Domené et al. 2008 developed sensitive assays of SIX3 function in zebrafish and confirmed that 89% of the deleterious mutations were loss-of-function alleles. Lacbawan et al. 2009 found that the more severe forms of holoprosencephaly demonstrated greater loss-of-function. The female/male ratio was 1.5:1 and maternal inheritance was twice as common as paternal inheritance. Fourteen percent of SIX3 mutations occur de novo. In familial cases, clinical variability is wide and penetrance is estimated to be at least 62%. The most commonly associated clinical findings in decreasing order of frequency are hypotelorism, microcephaly, cleft lip-palate, seizures, premaxillary agenesis, diabetes insipidus, single maxillary central incisor, and coloboma.
SIX3 mutations are summarized in Table XV together with the associated references. Ribeiro et al. 2006a reported one patient with a double SIX3 mutations (c.206G → A, resulting in c.Gly69Asp, and c.406dupGC, frameshift). Ming et al. 2002 and Domené et al. 2008 reported a double SIX3/PTCH1 mutation (SIX3: c.278C → A, resulting in p.Ala93Asp, and PTCH1: c.1165G → A, resulting in p.Ala393Thr).
| Mutations | Alterations |
|---|---|
| c.109G → T | p.Gly37Cys |
| c.206G → A, 406_407dupGC | c.Gly69Asp and FS |
| c.214G → C | p.Ala72Pro |
| c.235A → G | p.Met79Val |
| c.275T → G | p.Val92Gly |
| c.278C → A, PTCH1: c.1165G → A | p.Ala93Asp, p.Ala393Thr |
| c.311A → G | p.Asp104Gly |
| c.313A → G | p.Ile105Val |
| c.338G → A | p.Trp113Stop |
| c.339G → A | p.Trp113Stop |
| c.339G → T | p.Trp113Cys |
| c.341C → T | p.Ser114Leu |
| c.385G → T | p.Glu129Stop |
| c.389C → A | p.Ser130Stop |
| c.404G → C | p.Arg135Pro |
| c.404_407dupGC | FS |
| c.405_409dupCGCCG | FS |
| c.463_465CAC | p.His155del |
| c.469T → A | p.Phe157Ile |
| c.507delG | FS |
| c.515C → T | p.Ala172Val |
| c.518A → C | p.His173Pro |
| c.520T → C | p.Tyr174His |
| c.542_576dup | FS |
| c.551delC | FS |
| c.556_557dupGG | FS |
| c.582dupC | FS |
| c.605C → T | p.Thr202Ile |
| c.619G → T | p.Glu207Stop |
| c.637T → G | p.Phe213Val |
| c.652C → T | p.Arg218Trp |
| c.653G → C | p.Arg218Pro |
| c.676C → G | p.Leu226Val |
| c.680A → C | p.Gln227Pro |
| c.686C → T | p.Pro229Lys |
| c.692C → G | p.Pro231Arg |
| c.698_706del | p.Asn233-Ser235del |
| c.GC718_719AA | p.Ala240Lys |
| c.721C → T | p.Gln241Stop |
| c.730G → T | p.Gly244Cys |
| c.736delA | FS |
| c.743_745delCAG | p.Thr248LysdelGln |
| c.749T → C | p.Val250Ala |
| c.762T → A | p.Phe254Leu |
| c.769C → T | p.Arg257Trp |
| c.770G → C | p.Arg257Pro |
| c773G → C | p.Arg258Leu |
| c.785G → A | p.Arg262His |
| c.806G → C | p.Arg269Thr |
| c.806G → T | p.Arg269Met |
| c.806 + 1G → T | Splice |
| c.807GC | p.Arg269Ser |
| c.820_832delinsCTGGACCT | p.Ala274Stop |
| c.850G → C | p.Ala284Pro |
| c.890C → T | p.Pro297Leu |
| c.944C → T | p.Thr315Ile |
| Several microdeletions by qPCR | |
| del(2)(p2101p2109) | |
| del(2)(p21) | |
| del(2)p21p23) | |
| del(2)p16p22) | |
| del(2)(p21p22.2) | |
| del(2)(p21p22.1) | |
| ins(2)(p21q24q13) | |
| t(1;2)(p21;p21) | |
| t(1;2)(p22.3;p21) | |
| del(2)(p16.3p21) |
TGIF Mutations
TG-interacting factor (TGIF), which maps to 18p11.3, modulates TGFβ components that cause holoprosencephaly in animals. TGIF is an atypical homeodomain protein that is localized in the nucleus [Wallis and Muenke, 1999; Muenke and Cohen, 2000; Muenke and Beachy, 2001]. TGIF acts as a co-repressor of SMAD2/SMAD4 and in recruiting histone deacetylases to the complex [Wotton et al., 1999]. TGIF can also bind to the RXR binding site of the cellular retinol-binding protein II promoter (CRBPII RXRE) [Bertolino et al., 1995], but TGIF and RXR compete for binding to the promoter element [Bertolino et al., 1996]. Thus, TGIF can repress retinoic acid-regulated gene transcription. Perhaps TGIF mutations may produce loss of function as a repressor, resulting in overactivity of retinoic acid-regulated genes, simulating the effect of excessive retinoic acid exposure in humans [Lammer et al., 1985] and in mice [Webster et al., 1986; Sulik et al., 1988, 1995]. Helms et al. 1994, 1997 demonstrated an interrelationship between the SHH and retinoic acid pathways in the chick.
To date, at least six heterozygous missense mutations in TGIF have been identified in human holoprosencephaly [Gripp et al., 2000; Dubourg et al., 2004; Herbergs et al., 2002]. The mutation reported by Herbergs et al. 2002 was found in one parent who had hypotelorism and microcephaly.
PTCH1 Mutations
PTCH1 is part of the hedgehog signaling network, Helms et al. 1997 demonstrated that retinoic acid inhibited SHH and PTCH1 expression in the craniofacial primordia of chick embryos; the polarizing activity in these tissues was abolished. These findings are of particular interest because human mutations in both SHH [Roessler et al., 1996, 1997; Nanni et al., 1999] and PTCH1 [Ming et al., 1998] have been shown to cause holoprosencephaly.
PTCH1, which maps to 9q22.3, is a 12-pass transmembrane protein for which SHH is the ligand. PTCH1 mutations have been identified in patients with nevoid basal cell carcinoma syndrome, isolated basal cell carcinoma, medulloblastoma, meningioma, neuroectodermal tumor, breast cancer, squamous cell carcinoma of the esophagus, and trichoepithelioma [Cohen, 1999]. Most mutations have resulted in protein truncation. However, Ming et al. 1998 reported four PTCH1 missense mutations—two in extracellular loops that may be required for SHH binding, and two intracellular loops (Table XVI).
| Exons | Mutations | Amino acid substitutions | Domains | Phenotypes |
|---|---|---|---|---|
| Ribeiro et al. [2006b] | ||||
| 9 | 1316A → G | Ala443Gly | Intracellular | Lobar holoprosencephaly |
| 15 | 2240T → C | Val551Gly | Intracellular | Alobar holoprosencephaly |
| 17 | 2711T → G | Val908Gly | Extracellular | Alobar holoprosencephaly |
| 17 | 2711T → G | VL908Gly | Extracellular | Lobar holoprosencephaly |
| 18 | 3143C → T | Thr1052Met | Intracellular | Holoprosencephaly-like facial phenotype with normal brain MRI |
|
Ming et al. 2002 |
||||
| 8 | 1165G → A | Ala393Thr | Extracellular | Semilobar holoprosencephaly |
| 14 | 2171C → T | Thr728Met | Intracellular | Semilobar holoprosencephaly |
| 14 | 2171C → T | Thr728Met | Intracellular | Semilobar holoprosencephaly |
| 15 | 2471A → G | Ser827Gly | Extracellular | Holoprosencephaly |
| 18 | 3143C → T | Thr1052Met | Intracellular | Alobar holoprosencephaly |
Ribeiro et al. 2006b reported five probands with PTCH1 mutations (Table XVI) and highly variable phenotypes with holoprosencephaly in four cases and a holoprosencephaly-like phenotype in one case. Three mutations had not been reported previously (Ala443Gly, Val751Gly, andVal908Gly). Two patients had the same Val908Gly mutation but were phenotypically different: alobar holoprosencephaly, absent nasal septum, midline cleft lip-palate, and large ears in one case, and lobar holoprosencephaly, hypertelorism, clefting of the nose, severe bilateral microphthalmia, and single maxillary central incisor in the other. One patient had a Thr105Met mutation, holoprosencephaly-like facial features, and a normal MRI [Ribeiro et al., 2006b]. Ming et al. 2002 reported an identical mutation, but with alobar holoprosencephaly.
Mansilla et al. 2006 reported three instances of isolated cleft lip-palate with PTCH1 missense variants in probands. An unaffected relative or relatives had the same PTCH1 variant (Table XVII).
| Exon | PTCH1 missense changes in probands | Amino acid changes | PTCH1 missense changes in unaffected relatives |
|---|---|---|---|
| 6 | 895C → T | Pro295Ser | Two unaffected brothers and unaffected father |
| 9 | 1306G → A | Asp436Asn | Unaffected mother |
| 15 | 2479A → G | Ser827Gly | Unaffected mother |
- Adapted from Mansilla et al. with J. Murray as corresponding author [2006].
GLI2 Mutations
GLI2, which maps to 2q14 [Matsumoto et al., 1996] is part of the hedgehog signaling network. Transgenic mice overexpressing Gli2 in cutaneous keratinocytes developed multiple basal cell carcinomas [Grachtschouk et al., 2000]. Roessler et al. 2002 studied 300 familial and sporadic cases of holoprosencephaly. They identified seven sequence variations in GLI2. Functional studies showed four mutations with loss of function. Two mutations truncated the zinc finger domain. Another mutation was in the invariant splice donor consensus sequence. A frameshift mutation was found segregating in a large pedigree associated with panhypopituitarism.
Later, Roessler et al. 2005 reported a previously unidentified GLI2 sequence encoding an additional 328 amino acids at the amino terminus; their studies showed a strong inhibitory effect on transcriptional activity of wild-type GLI2 and established that this domain is essential for dominant-negative activity in GLI2 mutants. Roessler et al. 2005 noted that haploinsufficiency was associated in three affected families, whereas dominant-negative properties in the GLI2 mutant protein reduced transcriptional activity even further in two other affected families.
Rahimov et al. 2006 reported four patients with GLI2 mutations and their strikingly diverse phenotypes (Table XVIII), including one case with a double GLI2/PTCH1 mutation and a holoprosencephaly-like phenotype and another case with associated branchial arch anomalies.
| Gene | Nucleotide change | Amino acid substitution | Phenotype |
|---|---|---|---|
| Double mutation | Holoprosencephaly-like phenotype | ||
| GLI2 | 451A → G | Arg151Gly | |
| PTCH1 | 2171C → T | Thr328Met | |
| GLI2 | 1809C → T | Pro604Ser | Anophthalmia, branchial arch anomalies, CNS anomalies |
| GLI2 | 3348G → A | Met111Ile | Heminasal aplasia, orbital anomalies |
| GLI2 | 3677C → T | Pro1226Leu | Lobar holoprosencephaly |
- From Rahimov et al. 2006.
FOXH1 Mutations
FOXH1 maps to 8q24.3 [Zhou et al., 1998]. To assess the multiple hit hypothesis in the context of NODAL signaling, Roessler et al. 2008 examined the genomes of individuals with congenital heart defects, laterality, and holoprosencephaly for mutations in NODAL, GDF1, CFC1, TDGF1, FOXH1, and SMAD4. Gene products throughout the pathway were associated with conotruncal heart defects in about 5–10% of subjects, but far less commonly with isolated laterality (∼1%) or isolated holoprosencephaly (∼1%).
TDGF1 Mutations
TDGF1 (teratocarcinoma-derived growth factor 1) (CRIPTO), an epidermal growth factor (EGF)-cycteine-rich (CFC) family member, maps to 3p21-p23 [Ciccodicola et al., 1989; Saccone et al., 1995]. In mice, Tdgf1 is essential for germ and layer formation, positioning of the anteroposterior axis, and neural patterning [Shen and Schier, 2000]. EGF–CFC proteins are co-factors in Nodal signaling. They are membrane-associated, glycosylated, and have a characteristic EGF-like motif and CFC domain [Shen and Schier, 2000]. de la Cruz et al. 2002 screened 83 familial and 327 sporadic cases of holoprosencephaly. They identified two loss-of-function mutations in the CFC domain of TDGF1. One patient had semilobar holoprosencephaly and median orofacial clefting; the other had a dysplastic forebrain, hypoplastic corpus callosum, and absent septum pellucidum.
Fatty Liver in Holoprosencephaly
Fatty infiltration of the liver and other related liver alterations are common in patients with holoprosencephaly of at least five different etiologies, including SHH, SIX3, TGIF, FOXH1, and ZIC2 [Solomon et al., 2009a].
DHCR7 Mutations and Smith–Lemli–Opitz Syndrome
DHCR7 mutations cause Smith–Lemli–Opitz syndrome, an autosomal recessive disorder characterized by microcephaly, mental retardation, ptosis of the eyelids, epicanthic folds, strabismus, anteverted nares, micrognathia, cleft palate in some instances, characteristic Y-shaped 2–3 syndactyly, postaxial polydactyly, cardiovascular anomalies, and renal anomalies, together with ambiguous genitalia and cryptorchidism in males (Fig. 17). The syndrome is variably expressed from severe, such as holoprosencephaly in 5–6%, resulting in lethality, to minimal facial dysmorphism and mild mental impairment [Kelley et al., 1996; Gorlin et al., 2001; Nowaczyk et al., 2001; Steiner and Martin, 2007].
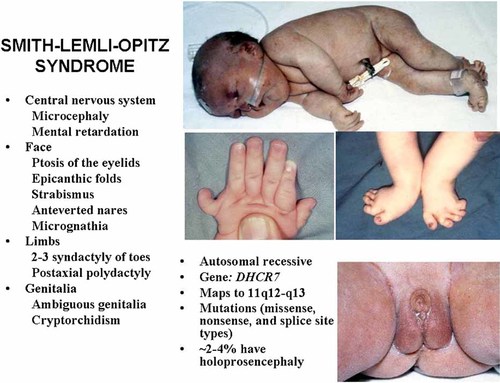
Smith–Lemli–Opitz syndrome montage. Full body and feet courtesy of D. Donnai, Manchester. From Gorlin et al. 2001.
DHCR7 maps to 11q12-q13. Mutations in DHCR7 (Δ7-sterol reductase) cause Smith–Lemli–Opitz syndrome. This results in an increased concentration of the precursor, 7-dehydrocholesterol with a reduced concentration in serum cholesterol (Fig. 13).
Mutations in the Smith–Lemli–Opitz syndrome are of the missense, nonsense, and splice site types. IVS8-1G > C has been identified in 29% of the patients [Kelley and Hennekam, 2000; Krakowiak et al., 2000; Witsch-Baumgartner et al., 2000; Yu et al., 2000a,b]. Nine cases of holoprosencephaly have been reported to date with molecular analysis in only two of them; one was homozygous for IV8-1G > C and one was a double mutation (IV8-1G > T and deletion of the entire 3rd and 4th exons) in a case of cyclopia [Weaver et al., 2010]. Although double mutations, such as for DHCR7 and, for example, SHH might be a possibility, this has not been reported to date. All known double mutations for holoprosencephaly have been reviewed in the section titled “Multiple Hit Hypothesis for Holoprosencephaly.”
Sterol depletion, but with still enough for the cholesterol moiety attachment to hedgehog may affect the activity of Smo [Cooper et al., 2003] (see section on Smoothened and Fig. 5). Willnow et al. 1996 showed that absence of megalin in knockout mice (Lrp−/Lrp−) produced holoprosencephaly (Table X). Dehart et al. 1997, using a rodent model for Smith–Lemli–Opitz syndrome, showed that cholesterol deficiency could interfere with embryonic plasma membrane function and cell-to-cell interaction.
The cholesterol supply during embryogenesis is an important factor affecting the Smith–Lemli–Opitz syndrome phenotype. Alipoprotein E (apo E), a constituent of lipoproteins in the plasma and body fluids, is involved in maternal–embryonal cholesterol transport. A simplified model of the role of apo E in this transport is shown in Figure 18. A genetic polymorphism in the apo E gene is characterized by three common alleles: 2, 3, and 4, which differ by base substitutions in two codon, resulting in alterations in two amino acid residues: 112 (Cys > Arg) and 158 (Arg > Cys). Witsch-Baumgartner et al. 2004 showed a significant correlation between clinical severity in Smith–Lemli–Opitz syndrome patients and apo E genotypes: a maternal apo 2 genotype was associated with a severe phenotype, whereas maternal apo E genotypes without the 2 allele were associated with a milder phenotype.
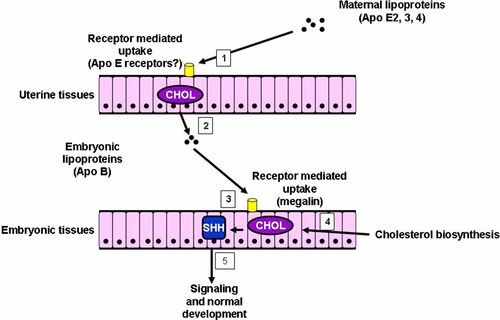
Simplified model of the possible role of apo E in maternal–embryonal cholesterol transport. In Smith–Lemli–Opitz syndrome patients with defective DHCR7, the embryo's cholesterol supply depends on the transport of lipoproteins from the mother. Apo E isoforms differ in their binding affinities to lipoprotein receptors, which make the efficiency of transport dependent on the maternal apo E genotype. For example, maternal apo E2 has been found to be associated with a severe phenotype, whereas apo E genotypes without E2 have been found to be associated with a milder phenotype. Modified and adapted from Witsch-Baumgartner et al. 2004.
The human blood–brain barrier closes to cholesterol transport early in embryonic gestation [Plotz et al., 1968]. If maternal–fetal cholesterol transport could be induced before closure of the blood–brain barrier, neurologic and behavioral abnormalities might be ameliorated. Recently, Jenkins et al. 2008 presented some data suggesting that high density lipoprotein (HDL) particles from Smith–Lemli–Opitz syndrome fetuses are better acceptors of cholesterol than HDL particles from normal fetuses, which raises the possibility that if more cholesterol could be transferred to the fetal circulation, the fetus would be capable of accepting it and circulating it in HDL particles. Although the role of maternal–fetal transport across the placenta has been controversial [Wollett, 2005], evidence supporting this pathway has been accumulating [Lindegaard et al., 2008].
The transfer of cholesterol between lipoproteins and cells depends on cholesterol transporters and receptors. ATP-binding cassette transporter Al (Abca1) is a cellular transporter protein that mediates the efflux of cholesterol from cells to lipid poor apo A1, contributing to the formation of HDL particles [Oram and Vaughn, 2000]. The ATP-binding cassette transporter G1 (Abcg1), belonging to the same family of transporters, appears to be involved in the efflux from cells also, but the acceptors of cholesterol are HDL2 and HDL3 particles [Wang et al., 2004]. Scavenger receptor class B type 1 (Sr-b1) is a multi-ligand receptor that mediates the cellular uptake of cholesterol from HDL particles, as well as the cholesterol efflux from cells [Acton et al., 1997; Xu et al., 1997; de la Llera-Moya et al., 1999; Thuahnai et al., 2004].
Abca1, Abcg1, and Sr-b1 mRNAs are all expressed in the placenta of the mouse [Christiansen-Weber et al., 2000; Hatzopoulos et al., 1998; Langmann et al., 2003]. The Smith–Lemli–Opitz syndrome mouse model is incapable of de novo synthesis of cholesterol in utero. Lindegaard et al. 2008 showed that Abca1 and possibly Sr-b1 contribute to transporting maternal cholesterol to the developing mouse fetus and suggest that modulating maternal–fetal transport has the potential for in utero therapy of human Smith–Lemli–Opitz syndrome.
Greig Cephalopolysyndactyly Syndrome and GLI3 Mutations
Greig cephalopolysyndactyly syndrome is an autosomal dominant disorder characterized by broad and prominent forehead; cutaneous syndactyly; postaxial polydactyly of the hands more commonly than preaxial polydactyly; and ocular hypertelorism with secondary telecanthus (Fig. 19) [Biesecker, 2004, 2006; Balk and Biesecker, 2008]. More severely affected patients may also have developmental delay, mental retardation, and on occasion, seizures and hydrocephalus, making up a variant disorder known as Greig cephalopolysyndactyly contiguous gene syndrome [Johnston et al., 2003, 2007]. At the mild end of the spectrum, some patients have the limb anomalies with either subtle or no craniofacial defects, showing that the boundary between syndromic and nonsyndromic preaxial polydactyly and cutaneous syndactyly is not clear [Biesecker, 2006].
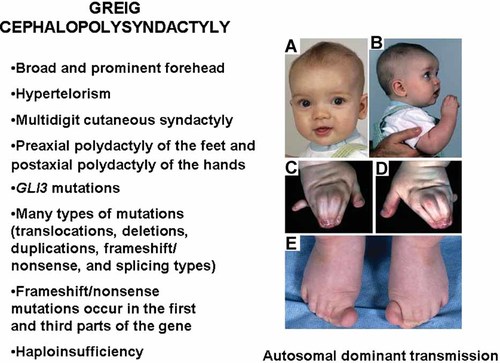
Greig cephalopolysyndactly syndrome montage. From Balk and Biesecker 2008.
GLI3 is a zinc-finger transcription factor gene which maps to 7p14.1. Greig syndrome and Pallister–Hall syndrome (vide infra) are allelic, both being caused by GLI3 mutations [Vortkamp et al., 1991; Kang et al., 1997; Wild et al., 1997]. Greig syndrome mutations are summarized in Table XIX [Johnston et al., 2005].
| Familial or sporadic | Exon | DNA alteration | Predicted protein alterations |
|---|---|---|---|
| Familial | 5 | c.540_547del8 | p.N181_T183delinCfsX15 |
| Sporadic | 5 | c.658delC | pR220VfsX3 |
| Familial | — | c.679 + 2_679 + 15del14 | IVSS |
| Familial | — | c.827−3C → G | IVS6 |
| Familial | 7 | c.868C → T | p.R290Xa |
| Sporadic | 8 | c.1048_1049insT | p.Y350LfsX2 |
| Familial | 8 | c.1074delC | p.H358QfsX7 |
| Sporadic | — | c.1497 + 1G → C | IVS10 |
| Familial | — | c.1497 + 1G → A | IVS10 |
| Familial | — | c.1497 + 1G → T | IVS10 |
| Familial | 10 | c.1446C → G | p.C482W |
| Familial | 11 | c.1617_1633del17 | p.R539_P545delinsSfsX7 |
| Sporadic | 12 | c.1789C → T | p.Q579X |
| Sporadic | 13 | c.1873C → T | p.R625W |
| Familial | 13 | c.1874G → A | p.R625Q |
| Sporadic | 13 | c.1880_1881delAT | p.H627RfsX62 |
| Familial | 14 | c.2374C → T | p.R792Xb |
| Sporadic | 15 | c.4119_4123delins7 | p.P1374_5137delinsAfsX2 |
| Sporadic | 15 | c.4402_4403insG | p.L1469AfsX10 |
| Sporadic | 15 | c.4427delA | p.S1477fsX11 |
| Familial | 15 | c.4564delG | p.A1522PfsX2 |
| Sporadic | 15 | c.4677_4678insC | p.R1560RfsX38 |
Acrocallosal syndrome, an autosomal recessive disorder characterized by polysyndactyly, macrocephaly, prominent forehead, hypertelorism, agenesis or dysgenesis of the corpus callosum, mental retardation, and/or developmental delay with or without seizures and CNS anomalies [Schinzel, 1982]. Distinction between Greig cephalopolysyndactyly syndrome and acrocallosal syndrome in sporadic instances can be challenging, but demonstrating haploinsufficiency for GLI3 through molecular testing unambiguously identifies Greig syndrome and excludes acrocallosal syndrome [Johnston et al., 2003].
Pallister–Hall Syndrome and GLI3 Mutations
Characteristic features that distinguish Pallister–Hall syndrome from Greig cephalopolysyndactyly syndrome include hypothalamic hamartoma, bifid epiglottis, and insertional polydactyly (Fig. 20) [Biesecker, 2006]. Other findings include osseous syndactly and may include seizures, precocious puberty, panhypopituitarism or less commonly growth hormone deficiency, laryngeal cleft, atypical lung lobation, and imperforate anus. The disorder has autosomal dominant inheritance with great variability in phenotypic expression from mild to severe. Patients at the mild end of the phenotypic spectrum are often familial. Sporadic cases may be mild, moderate, or severe. A Pallister–Hall syndrome patient with any of the following medical problems—laryngeal cleft, adrenal insufficiency, or anal atresia—constitute a medical emergency. Immediate securing the airway is the first priority if respiratory distress is present, which can be caused by a laryngeal cleft, whereas the diagnostic feature, bifid epiglottis is symptom free. The most important emergency with panhypopituitarism is adrenal insufficiency and should be treated if present. Imperforate anus with its subsequent bowel obstruction needs immediate attention [Biesecker, 2004].
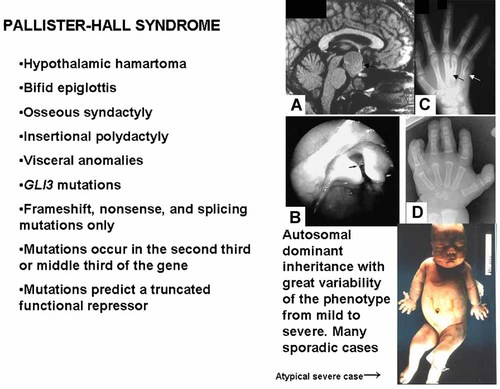
Pallister–Hall syndrome montage. A: Hypothalamic hamartoma. B: Bifid epiglottis. C: Insertional polydactyly. D: Polydactyly. Clinical phenotype here is atypical and severe. From Graham et al. 1986.
GLI3 mutations that cause Pallister–Hall syndrome and Greig cephalopolysyndactyly syndrome are distinct in two ways. First, the mutations causing Greig syndrome include translocations, large deletions, frameshift, missense, nonsense, and splice site mutations (Table XIX). In contrast, mutations in Pallister–Hall syndrome are almost completely limited to frameshift and nonsense mutations (Table XX). Second, the positions of the frameshift mutations in the two disorders are distinctive. Mutations in the first third of the gene cause Greig syndrome, whereas mutations in the second-third of the gene cause primarily Pallister–Hall syndrome [Johnston et al., 2005; Biesecker, 2006]. Thus, it is clear that these two allelic disorders have different modes of pathogenesis: functional haploinsufficency causes Greig cephalopolysyndactyly syndrome and a truncated functional repressor causes Pallister–Hall syndrome [Johnston et al., 2005; Biesecker, 2006].
| Familial or sporadic | Exon | DNA alteration | Predicted protein alterations |
|---|---|---|---|
| Familial | 13 | c.1998_2001del4 | p.P668_T669delinsLfsX24 |
| Familial | 13 | c.2032delG | p.D678fsX15 |
| Sporadic | 13 | c.2062G → T | p.E688X |
| Sporadic | 14 | c.2110C → T | p.Q704X |
| Sporadic | 14 | c.2139delC | p.C713fsX13 |
| Sporadic | 14 | c.2146C → T | p.Q716X |
| Sporadic | 14 | c.2149C → T | p.Q717X |
| Familial | 14 | c.2157delC | p.1720SfsX13 |
| Familial | 14 | c.2172_2173insC | p.N725QfsX13 |
| Sporadic | 14 | c.2188_2207del20 | p.L730X |
| Familial | 14 | c.2197_2198delAC | p.T733RfsX4 |
| Familial | 14 | c.2346_2356del11 | p.R82_V786delinsSfsX15 |
| Sporadic | 14 | c.2351_2355del5 | p.K784_Q785delinsSfsX15 |
| Familial | — | c.2431 + 1G → A | IVS14 |
| Sporadic | 15 | c.283delC | p.P828RfsX14 |
| Sporadic | 15 | c.2567C → A | p.S856X |
| Sporadic | 15 | c.2620delC | p.R874AfsX16 |
| Sporadic | 15 | c.2628delC | p.S877AfsX13 |
| Sporadic | 15 | c.2770_2771ins72 | p.A924delins(12)X |
| Sporadic | 15 | c.2799C → G | p.Y933X |
| Sporadic | 15 | c.3324C → G | p.Y1108X |
| Familial | 15 | c.3386_3387delT | p.F1129X |
| Sporadic | 15 | c.3456G → T | p.E1152X |
- Adapted from Johnston et al. 2005.
Craig et al. 2008 identified somatic chromosomal abnormalities with loss of heterozygosity within GLI3 in 15% of isolated (non-Pallister–Hall) hypothalamic hamartomas (n = 55).
Carpenter Syndrome and RAB23 Mutations
Carpenter syndrome is an autosomal recessive disorder characterized by craniosynostosis, most commonly involving the sagittal and metopic sutures; short fingers with clinodactyly; commonly but not always preaxial polydactyly of the feet; sometimes postaxial polydactyly; and other abnormalities, such as congenital heart defects, short stature, obesity, and mental deficiency (Fig. 21) [Gorlin et al., 2001]. Carpenter syndrome is caused by RAB23 mutations [Jenkins et al., 2007] (Table XXI).

Carpenter syndrome montage. From Jenkins et al. 2007 and courtesy of A.O.M. Wilkie.
| Family | Sex | Parental consanguinity | Mutations | |||
|---|---|---|---|---|---|---|
| Maternal | Paternal | |||||
| DNA | Protein | DNA | Protein | |||
| M | — | 140-141insA | E48fsX7 | 140-141insA | E48fsX7 | |
| F | First cousin | 232delT | Y78fsX30 | Samea | — | |
| F | — | 434T → A | L145X | 253T → C | 85R | |
| F | — | 408 409insT | E137X | 408 409insT | E137X | |
| F | First cousin once removed | 408 409insT | E137X | Samea | — | |
| Family 1 | M | — | 434T → A | L145X | 434T → A | L145X |
| Family 1 | F | — | 434T → A | L145X | 434T → A | L145X |
| Family 1 | M | — | 434T → A | L145X | 434T → A | L145X |
| Family 2 | M | First cousin | 434T → A | L145X | Samea | — |
| F | — | 434T → A | L145X | 434T → A | L145X | |
| M | — | 434T → A | L145X | 434T → A | L145X | |
| M | First cousin | 434T → A | L145X | Samea | — | |
| M | First cousin | 434T → A | L145X | Samea | — | |
| F | — | 434T → A | L145X | 434T → A | L145X | |
| F | — | 434T → A | L145X | 434T → A | L145X | |
| M | First cousin | 434T → A | L145X | Samea | — | |
| M | — | 434T → A | L145X | 434T → A | L145X | |
- Adapted from Jenkins et al. with A.O.M. Wilkie as corresponding author [Jenkins et al., 2007].
- a For cases in which the two parental alleles are very unlikely to be independent due to known consanguinity, the paternal allele is denoted “Same.”
Rubinstein–Taybi Syndrome and CBP Mutations
Rubinstein–Taybi syndrome is characterized by growth deficiency, mental retardation, microcephaly, downslanting palpebral fissures, curved nose in profile with the septum extending below the alae nasi, mild micrognathia, broad thumbs, and broad great toes (Fig. 22). Many other abnormalities have been reported including, among others, strabismus (58%), heart defects (32%), and various tumors (nasopharyngeal rhabdomyosarcoma, intraspinal neurilemoma, neuroblastoma, meningioma, and pheochromocytoma [Gorlin et al., 2001].
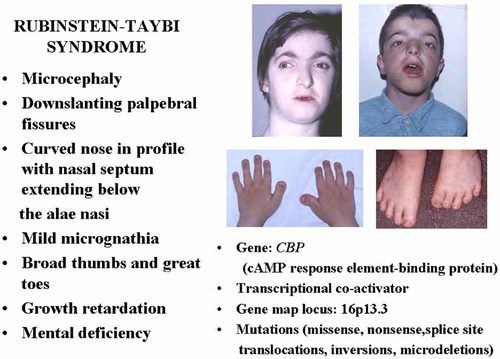
Rubinstein–Taybi syndrome montage.
CBP,2 which maps to 16p13.3, is a transcriptional co-activator involved in different signal transduction pathways that regulates the expression of many genes and plays an important role in the regulation of cell growth, cellular differentiation, and tumor suppression. CBP mutations cause Rubinstein–Taybi syndrome [Petrij et al., 1995; Coupry et al., 2002]. Microdeletions in CBP have been found in about 15% of patients [Gorlin et al., 2001]. Missense mutations, nonsense mutations, and splice site mutations have also been identified [Coupry et al., 2002; Bartsch et al., 2005; Schorry et al., 2008] (Table XXII).
| Type of mutation | Exon | Mutations |
|---|---|---|
| Nonsense | 1 | Ser23Stop |
| 2 | 138delA + ins13 bp | |
| 3 | 840insT | |
| 4 | Arg370Stop | |
| 5 | Arg413Stop | |
| 10 | 2045insA | |
| 14 | 2827delC | |
| 16 | 3096insT | |
| 21 | Lys1269Stop | |
| 27 | Tyr1466Stop | |
| 27 | Arg1498Stop | |
| 30 | 4945delA | |
| 31 | Gln2043Stop | |
| Missense | 15 | Arg981Thr |
| 31 | Asn1978Ser | |
| 31 | Met2221Leu | |
| 31 | Ala2243Val |
| Splice site | Intron | Mutations |
|---|---|---|
| 17 | del17 bp + ins2 bp | |
| 19 | IVS19+3A → T | |
| 25 | IVA25+2T → C | |
| 27 | IVS27−5C → G | |
| 27 | Lys1520Arg |
- Data from Coupry et al. 2002.
Schorry et al. 2008 studied genotype–phenotype correlations with mutation types: truncating large deletions, single amino acid substitutions, or no CBP mutations. Growth deficiency in height and weight were observed more frequently in patients with no mutation. A trend towards lower IQ was found in patients with large deletions. Rubinstein–Taybi syndrome is etiologically heterogeneous. Some cases without CBP mutations have been found to have EP300 mutations and preliminary evidence has shown that some cases may lack broad thumbs. Other patients with Rubinstein–Taybi syndrome may be caused by an as yet unidentified gene [Schorry et al., 2008].




