Mutation analysis of B3GALTL in Peters Plus syndrome†
How to cite this article: Reis LM, Tyler RC, Abdul-Rahman O, Trapane P, Wallerstein R, Broome D, Hoffman J, Khan A, Paradiso C, Ron N, Bergner A, Semina EV. 2008. Mutation analysis of B3GALTL in Peters Plus syndrome. Am J Med Genet Part A 146A:2603–2610.
Abstract
Peters Plus syndrome comprises ocular anterior segment dysgenesis (most commonly Peters anomaly), short stature, hand anomalies, distinctive facial features, and often other additional defects and is inherited in an autosomal-recessive pattern. Mutations in the β1,3-glucosyltransferase gene (B3GALTL) were recently reported in 20 out of 20 patients with Peters Plus syndrome. In our study, B3GALTL was examined in four patients with typical Peters Plus syndrome and four patients that demonstrated a phenotypic overlap with this condition. Mutations in B3GALTL were identified in all four patients with typical Peters Plus syndrome, while no mutations were found in the remaining four patients that demonstrated some but not all characteristic features of the syndrome. The previously reported common mutation, c.660 + 1G > A, accounted for 75% of the mutant alleles in our Peters Plus syndrome population. In addition, two new mutant alleles, c.459 + 1G > A and c.230insT, were identified and predicted to result in truncated protein products. These data confirm an important role for B3GALTL in causing typical Peters Plus syndrome, and suggest that this gene may not be implicated in syndromic cases that involve Peters anomaly but lack other classic features of this complex condition. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Peters Plus syndrome is a variable disorder with autosomal-recessive inheritance involving ocular and systemic abnormalities. The most common ocular feature is Peters anomaly (73% of patients), a developmental defect characterized by the triad of central corneal opacity, defects in the posterior layers of the cornea, and lenticulo-corneal and/or irido-corneal adhesions [Peters, 1906; Heon et al., 1992]. A second group (25% of patients) is affected with a different anterior chamber anomaly (such as Axenfeld-Rieger anomaly, sclerocornea, and posterior embryotoxon) and the remaining 2% have other congenital eye malformations [Maillette de Buy Wenniger-Prick and Hennekam, 2002]. Ocular anomalies are frequently bilateral, but can be unilateral.
In addition to the ocular abnormalities, the following systemic features are commonly seen in Peters Plus syndrome: short stature, short broad hands with fifth finger clinodactyly, distinctive facial features, cleft lip and/or cleft palate, hearing loss, abnormal ears, heart defects, genitourinary anomalies, variable degrees of mental retardation, and central nervous system abnormalities, including hydrocephalus [Maillette de Buy Wenniger-Prick and Hennekam, 2002] (Table I). Characteristic facial features include a cupid bow upper lip, round face, thin upper lip, long philtrum, hypertelorism, short palpebral fissures, prominent forehead, and broad neck [Thompson et al., 1993; Maillette de Buy Wenniger-Prick and Hennekam, 2002]. Ears are typically low-set and small with preauricular pits/cysts. Reported genitourinary anomalies include renal duplication, hydronephrosis, hypospadias, cryptorchidism, incomplete foreskin, and hypoplastic clitoris and labia majora [Maillette de Buy Wenniger-Prick and Hennekam, 2002].
| Patient/study reported | B3GALTL genotype | Peters anomaly | Anterior chamber anomaly | Short stature (≤3rd) | Brachydactyly | Developmental delay | Cleft lip/palate | Heart anomaly | GU anomaly | Ear anomaly | Pregnancy loss in mother |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1/this study | Homozygous c.660 + 1G- > A | B | + | + | + | + | B L/P | + | + | Small, posteriorly rotated | + |
| Patient 2/this study | Homozygous c.660 + 1G- > A | B | + | + | + | + | B L/P | − | − | Low-set, U ear pit | − |
| Patient 3/this study | c.660 + 1G > A; c.459 + 1G- > A | B | + | + | + | + | CP | + | − | Low-set | + |
| Patient 4/this study | c.660 + 1G > A; c.230insT | B | + | + | + | Unknown | U CL | + | + | − | − |
| Patient 5/this study | Negative | U | + | − | − | + | − | − | + | B ear creases | − |
| Patient 6/this study | Negative | B | + | − | NR | Unknown | − | − | + | Low-set | − |
| Patient 7/this study | Negative | − | + | + | − | + | CP | + | + | Low-set | + |
| Patient 8/this study | Negative | U | + | − | + | − | B L/P | − | − | B ear tags | − |
|
Patients 1–20; Lesnik Oberstein et al. 2006 |
20/20 mutateda | 15/19 | 20/20 | 20/20 | NR | 15/19 | 9/20 | 5/19 | Renal 5/20 | NR | NR |
|
NR; Maillette de Buy Wenniger-Prick and Hennekam 2002 |
NR | 73% | 98% | 92% | 100% | 83% | CL 45%; CP 33% | 31% | Renal 19% | Small 42%; ear pits 37% | + (freq NR) |
- NR = not reported, B = bilateral, U = unilateral.
- a Represents 15 independent cases and 5 affected siblings. Patients in 13/15 unrelated families were homozygous for the c.660 + 1G > A mutation; 2/15 were compound heterozygotes—c.660 + 1G > A; 1.5 Mb deletion and c.660 + 1G > A; c.347 + 5G > A.
B3GALTL (B3GTL) is located at 13q12.3 and was originally identified by Heinonen and collaborators and was later found to encode a glucosyltransferase [Heinonen et al., 2003; Kozma et al., 2006; Sato et al., 2006; Hess et al., 2008]. Glycosyltransferases play important roles in various biological processes and encode enzymes that catalyze the attachment of a sugar molecule to specific acceptor sites [Ohtsubo and Marth, 2006]. The β1,3-glucosyltransferase is involved in synthesis of the disaccharide Glc-β1,3-Fuc-O- that occurs on thrombospondin type 1 repeats (TSRs) of many biologically important molecules [Adams and Tucker, 2000; Silverstein, 2002; Hess et al., 2008]. The B3GALTL transcripts were shown to be present in numerous human and mouse tissues with the heart, kidney, and brain being major sites of expression in both species [Heinonen et al., 2003, 2006].
The role of B3GALTL in Peters Plus syndrome was discovered by Lesnik Oberstein et al. 2006 following identification of a 1.5 Mb interstitial deletion of one copy of chromosome 13 containing B3GALTL in two affected brothers. The other allele had a c.660 + 1G > A mutation in exon 8 in both brothers. Sequencing in an additional 14 probands showed that 13 were homozygous for the same point mutation, while the remaining proband was a compound heterozygote for c.660 + 1G > A and c.347 + 5G > A. Interestingly, significant clinical heterogeneity was noted within the reported patient population [Lesnik Oberstein et al., 2006].
The splicing B3GALTL mutations described above were predicted to result in truncated protein products lacking the catalytic domain. These findings taken together with the presence of the B3GALTL deletion in the two original patients suggested that Peters Plus syndrome is likely to be due to a complete loss of functional B3GALTL protein [Lesnik Oberstein et al., 2006]. Another recent study demonstrated that synthesis of the disaccharide Glc-β1,3-Fuc-O- is disrupted in patients with homozygous B3GALTL mutations in contrast with their heterozygous relatives [Hess et al., 2008]. These data clearly established Peters Plus syndrome as a congenital disorder of glycosylation.
We undertook screening of B3GALTL in a population of eight patients, four of which had typical Peters Plus syndrome while the other four had some but not all features of this condition. This study was aimed at confirming the association of B3GALTL with Peters Plus syndrome, determining whether mutations in other regions of the gene should be considered, and exploring the possibility that some similar phenotypes may also be explained by mutations in B3GALTL.
CLINICAL REPORTS
Four patients with typical Peters Plus syndrome diagnosed by the referring physician (Patients 1–4) and four patients with features overlapping the Peters Plus phenotype (Patients 5–8) were enrolled into the study.
Patient 1
This Caucasian female was born at 33 weeks gestation with a birth weight of 1.49 kg (10th–25th centile) and length of 41 centimeters (10th–25th centile). The pregnancy was complicated by identification of cleft lip and palate by ultrasound and heart anomaly by fetal echocardiogram, as well as polyhydramnios noted at the beginning of the third trimester. Chromosomal analysis was normal (550 band level) and 22q11.2 fluorescent in situ hybridization (FISH) analysis was normal. At seven weeks of age, her weight was 2.3 kg (<3rd centile) and length was 43 cm (<3rd centile), with a head circumference of 33.5 cm (∼25th centile). At 13 months of age, her failure to thrive persisted with a weight of 7.3 kg (<3rd centile) and a length of 68 cm (<3rd centile). The patient had bilateral Peters anomaly with corneal opacification and lenticular-corneal adhesions noted, as well as a retinal coloboma in the left eye. In addition to bilateral cleft lip and palate, she was described to have an asymmetric face with fine eyebrows, narrow eyes with upslanting palpebral fissures, a prominent forehead, short neck, and apparently small, posteriorly rotated ears. Her hands and feet were apparently short and wide with fifth finger clinodactyly and apparent rhizomelic limb shortening. The patient had a mild atrial septal defect and her anus was anteriorly placed. Gastrointestinal malrotation was diagnosed and surgically repaired at 4 months of age. Apparently small labia minora and clitoris were noted at 7 months of age. Her development was noted to be delayed at 13 months of age; magnetic resonance imaging (MRI) of the brain showed possible intracranial calcifications, but no other anomalies. There was a history of one previous miscarriage and one pregnancy termination for these parents, with no medical details available regarding either. The mother also had micrognathia, a high-arched palate, and fifth finger clinodactyly. There was no evidence of consanguinity.
Patient 2
This Hispanic female was born at 36 weeks gestation with a birth weight of 2.40 kg (∼25th centile). Chromosomal analysis (700 band level) and subtelomeric FISH studies were normal. At 8 years of age, the patient's weight and height were 18.5 kg (<3rd centile) and 110 cm (<3rd centile) respectively, and head circumference was 50 cm (∼10th centile). She had bilateral Peters anomaly with corneal opacity and sclerocornea and a cataract in the right eye; she underwent a corneal transplant in the left eye. The patient had bilateral cleft lip and palate, micrognathia, and widely spaced teeth. She was described to have an asymmetric head shape with a prominent forehead, broad asymmetric nose, long philtrum, maxillary hypoplasia, apparent hypertelorism, and low-set ears with an ear pit in the antihelix of the left ear. The patient had apparent rhizomelic limb shortening, apparent brachydactyly of the fingers with clinodactyly and fetal finger pads, and apparently short feet and toes. In addition, she had borderline sensorineural hearing loss. Cranial MRI demonstrated partial agenesis of the corpus callosum and mild dilation of the lateral ventricles involving the occipital horns and atria (colpocephaly). Development was delayed with the patient being held back a year in first grade and currently receiving special education services. There were two healthy full siblings. The family originates from El Salvador; there was no evidence of consanguinity (Fig. 1A–C).
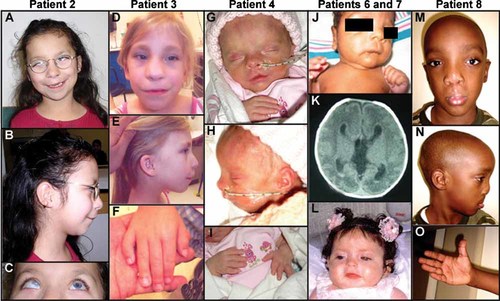
Patient photographs. Patient 2 with prominent forehead, asymmetric face, long philtrum, thin lips, maxillary hypoplasia, apparent hypertelorism, and low-set ears (A,B). Note corneal clouding in the right eye typical of Peters anomaly (left eye status post corneal transplant) (C). Patient 3 with long philtrum, thin lips, macrostomia, maxillary hypoplasia, high nasal bridge, prominent forehead, upsplanting palpebral fissures (D), and low-set, posteriorly rotated ears (E). Her hands show brachydactyly and fifth finger clinodactyly (F). Patient 4 had left-sided incomplete cleft lip, apparent hypertelorism, high nasal bridge, and micrognathia (G,H). Her hands show bilateral brachydactyly (I). Patient 6 with apparent hypertelorism, uneven palpebral fissures, thin lips, micrognathia, low-set ears, and widely spaced nipples (J). Head CT shows hydrocephalus with dilation of the lateral and third ventricles as well as agenesis of the corpus callosum (K). Patient 7 with thin upper lip, flat philtrum, small mouth, apparent hypertelorism, and prominent forehead (L). Patient 8 with apparent hypertelorism, high nasal bridge, wide flat nasal tip with asymmetric alae, and low-set ears (M,N). Hands are apparently short with brachydactyly (O). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient 3
This Caucasian female was born at 33 weeks gestation with a birth weight of 1.49 kg (10th–25th centile). Chromosomal analysis showed normal karyotype (750 band level). At 9 years of age, her weight was 20 kg (<3rd centile) with a height of 119 cm (<3rd centile). The patient had bilateral Peters anomaly with corneal opacity and irido-corneal adhesions, bilateral cataracts and glaucoma; she underwent bilateral corneal transplants, but is legally blind in both eyes. She had cleft palate and was described to have micrognathia, maxillary hypoplasia, a long philtrum with thin upper lip, broad nasal bridge, broad forehead, upsplanting palpebral fissures, and low-set ears. The patient had apparent brachydactyly, bilateral fifth finger clinodactyly, and short stature without rhizomelia. In addition, she had a ventricular septal defect and hearing loss diagnosed by audiometry. Her development was reported to be moderately to severely delayed. Cranial imaging was recommended but declined. There was one healthy full sibling and a history of a pregnancy termination due to hydrocephaly and polycystic kidneys noted in the fetus. There was no evidence of consanguinity (Fig. 1D–F).
Patient 4
This Caucasian female was born at 36–37 weeks gestation with a birth weight of 1.82 kg (∼3rd centile) and a length of 39.5 cm (<3rd centile). The pregnancy was complicated by diagnosis on ultrasound of intrauterine growth restriction (IUGR), ventriculomegaly, shortened long bones, and a possible heart defect. Chromosomal analysis in the neonatal period was normal (650 band level). At three weeks of age, her weight was 2.10 kg (<3rd centile) and length was 43 cm (<3rd centile). At 5 months of age, her failure to thrive persisted with a weight of 4.0 kg (<3rd centile) and length of 55.5 cm (<3rd centile). The patient was affected with bilateral Peters anomaly with corneal opacities and irido-corneal adhesions noted. She had a left-sided incomplete cleft lip and apparent hypertelorism, a high nasal bridge, and micrognathia with normally formed and positioned ears. The patient had clinodactyly of the left hand only and apparent brachydactyly of the hands and feet, along with short broad ribs and an apparently narrow chest. On echocardiogram at 1 day of age, the patient was noted to have a thickened pulmonary valve with mild pulmonary stenosis, a large patent ductus arteriosus, and a small atrial septal defect or patent foramen ovale. A renal ultrasound showed small kidneys which were hyperechoic and slightly edematous with increased resistive indices. Cranial MRI revealed hydrocephalus with enlargement of lateral ventricles, mild prominence of the third ventricle, and a thin corpus callosum. The patient also had widely spaced nipples and an anteriorly placed anus. A gastric tube was placed due to history of poor feeding. This was the first pregnancy to these parents; there was no evidence of consanguinity (Fig. 1G–I).
Patient 5
This Korean male was born at 36 weeks gestation with a birth weight of 4.35 kg (>97th centile). The pregnancy was complicated by identification of increased nuchal thickness and kidney dilation on ultrasound. Chromosomal analysis (550 band level), subtelomeric FISH, and 22q11.2 FISH studies in the neonatal period were normal. At 1 year of age, weight, and height were 11.2 kg (∼75th centile) and 78 cm (∼75th centile) respectively, with head circumference of 49.5 cm (>95th centile). The patient had a diagnosis of unilateral Peters anomaly and bilateral nystagmus. He was described to have mild facial asymmetry, slight tenting with down-turned corners of the mouth, a thin upper lip, broad neck, and bilateral creases of the ear lobes. He also had a flat occiput and tower-shaped skull, felt to be positional in nature; no other abnormal skeletal features were reported. In addition, the patient had a small foreskin of the penis and bilateral hydroceles of the testes. At 1 year of age, he had severe hypotonia and absence of language development. Cranial imaging by computed tomography (CT) scan was normal. Family history revealed one healthy full sibling and macrocephaly in the father and paternal grandfather. There was no evidence of consanguinity.
Patient 6
This Black male (of Jamaican decent) was born at 33 weeks gestation with a birth weight of 1.72 kg (25th–50th centile) and length of 41.5 centimeters (10th–25th centile). Chromosomal analysis in the neonatal period showed normal karyotype (550–600 band level). At 2 weeks of age, his weight was 2.68 kg (50–75th centile), length was 43 cm (∼10th centile), and head circumference was 34 cm (75–90th centile). The patient had bilateral Peters anomaly with significant corneal opacity prohibiting evaluation of the anterior chamber and elevated intraocular pressure bilaterally. He was described as apparently hyperteloric with thin lips, a high arched palate with micrognathia, facial hypertrichosis, and low-set ears. His hands were held clenched bilaterally; no abnormal skeletal features were reported. In addition, the patient had bilateral cryptorchidism and apparently widely spaced nipples. Cranial CT showed hydrocephalus with dilation of the lateral and third ventricles, agenesis of the corpus callosum, and multiple hyperdense foci along the margin of the third ventricle and within bilateral occipital horns, consistent with the presence of subependymal and intraventricular hemorrhage. There were three healthy maternal half-siblings but this was the first pregnancy to these parents. There was no evidence of consanguinity (Fig. 1J,K).
Patient 7
This Hispanic female was born at 38–39 weeks gestation with a birth weight of 2.79 kg (25th centile) and length of 46 cm (10–25th centile). The pregnancy was complicated by mild oligohydramnios and elevated alpha-fetoprotein on maternal serum screening. Prenatal ultrasound revealed mild renal dilation, an echogenic intracardiac focus, and a cleft in the inferior portion of the cerebellum with communication between the cisterna magna and lower fourth ventricle. Chromosomal analysis (band resolution unknown) and 22q11.2 FISH studies in the neonatal period were normal. At 4 months 3 weeks of age, her weight and length were 5.6 kg (∼10th centile) and 57 cm (<3rd centile) respectively, with a head circumference of 37.5 cm (<3rd centile). The patient had bilateral corneal opacities with elevated intraocular pressure (28, 41 mmHg), buphthalmos, glaucoma, and bilateral 360° posterior embryotoxon. She had a cleft palate and was described to have a cupid bow upper lip, thin upper lip, maxillary hypoplasia, short philtrum, small mouth, apparent hypertelorism with prominent eyes, frontal bossing, and low-set ears. Her hands and feet appeared normal except for fifth finger clinodactyly. The patient had a patent ductus arteriosus which required ligation, bicuspid aortic valve, bilateral hydronephrosis, renal reflux, and mild renal dilation. She also had bilateral congenital hip dysplasia, apparent widely spaced nipples, laryngomalacia, gastroesophageal reflux, and had a gastric tube placed due to a history of poor feeding. The patient has seizures, hypotonia, and delayed milestones (at a 2 month-level developmentally at 5 months chronological age). Cranial CT showed a poorly developed corpus callosum and slight prominence of CSF space posterior to the cerebellum (probable Dandy–Walker variant). The parents had a history of one previous miscarriage. The father is from Mexico and the mother is from Peru; there was no evidence of consanguinity (Fig. 1L).
Patient 8
This African-American male was born at 42 weeks from a twin gestation with a birth weight of 1.93 kg (<3rd centile). Prenatal ultrasounds were normal. At 5 years of age, his height was 115 cm (∼90th centile) and head circumference was 55 cm (>95th centile). The patient had Peters anomaly of the left eye with corneal opacity and irido-corneal adhesions, left microphthalmia, and left corectopia but the right eye was normal. He had bilateral cleft lip and palate and was described to have apparent hypertelorism with upslanting palpebral fissures, cupid bow upper lip, apparently wide, flat nasal bridge, asymmetric alae, and bilateral ear tags. His hands were apparently short; his feet were normal. There was no rhizomelic limb shortening. Cranial CT and MRI at 4 years of age showed hydrocephalus which was treated with a ventriculoperitoneal shunt. The patient had a history of two febrile seizures. His developmental milestones were normal. There were two healthy full siblings, including the patient's twin. There was no evidence of consanguinity (Fig. 1M–O).
MATERIALS AND METHODS
This human study was approved by the Institutional Review Board of the Children's Hospital of Wisconsin. Blood or saliva samples from each patient were collected and processed into DNA by standard methods. B3GALTL exons and flanking introns were amplified using PCR primers and conditions that are summarized in supporting information Table II (supporting information Table II may be found in the online version of this article). Direct sequencing of the PCR products was performed using ABI Sequencer equipment and protocols. The sequences were analyzed both manually and by using VectorNTI software to identify heterozygous and/or homozygous changes in B3GALTL in affected individuals in comparison to normal controls. To determine normal variation in B3GALTL, 180 samples obtained from the European Collection of Cell Cultures (HRC panels 2 and 3) were examined using the same protocols.
RESULTS
Since the previous report [Lesnik Oberstein et al., 2006] demonstrated the presence of only two mutations in B3GALTL (in exons 5 and 8) in patients with Peters Plus syndrome, we began our search by screening these exons. Patients 1 and 2 appeared to be homozygous for the previously reported c.660 + 1G > A mutation in exon 8 (Fig. 2). Patients 3 and 4 were heterozygous for c.660 + 1G > A. Screening of the remaining thirteen B3GALTL exons and their flanking regions in all patients revealed that Patient 3 had a second mutation in exon 6, c.459 + 1G > A (Fig. 2), and Patient 4 had a c.230insT mutation in exon 4 (Fig. 2). The c.459 + 1G > A mutation is predicted to lead to a truncated protein product or nonsense mediated decay (NMD). The c.230insT predicts p.Leu77PhefsX27, which may also induce NMD (see http://www.hgvs.org/).
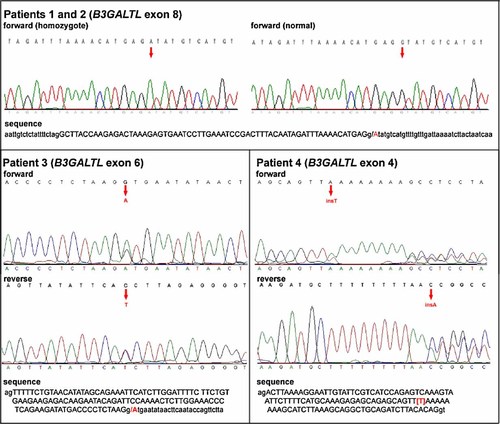
B3GALTL mutations in Peters Plus syndrome patients. Sequencing chromatograms obtained for Patients 1–4 are shown (please see text for details); nucleotide sequences of corresponding regions are also provided (intron sequences are shown in lower case while exon sequences in upper case). The sites of the mutations are indicated by red letters and arrows. Mutations in exons 4 and 6 represent novel alterations in B3GALTL. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Therefore four of four patients with typical Peters Plus syndrome had mutations in B3GALTL. No causative mutations in B3GALTL were identified in Patients 5–8 that demonstrated some but not all features of the Peters Plus phenotype (Table I).
Parental samples were available for Patients 2 and 4; in both cases, the parents were found to be heterozygous carriers for the mutations seen in their child. Since parental samples were unavailable for Patient 1, the c.660 + 1G > A mutation homozygous status could not be confirmed. An alternative possibility is that this patient carries the c.660 + 1G > A mutation in one allele and a B3GALTL deletion in the other allele.
Screening of 180 control samples demonstrated absence of the above reported sequence alterations in unaffected individuals. Several polymorphisms were identified in B3GALTL that were present in the patients and control individuals. Identified polymorphisms include c.459 + 66A > T, c.506 + 50delC, c.776 + 58A > G, c.978-88G > C and 3'UTR variants c.1520G > T and c.1527A > G.
DISCUSSION
We report the previously described (c.660 + 1G > A) as well as two novel B3GALTL mutant alleles (c.230insT and c.459 + 1G > A) in patients with typical Peters Plus syndrome. The two new mutations are predicted to be of similar severity to those previously reported [Lesnik Oberstein et al., 2006]. The c.459 + 1G > A mutation is predicted to alter splicing while c.230insT is expected to result in a frameshift, both leading to truncated protein products or NMD. Lesnik Oberstein et al. 2006 investigated the origin of the common mutation by analyzing three intragenic SNPs in the B3GALTL gene in their homozygous population, including three Dutch sibling pairs. Heterozygosity of at least one of the three SNPs was reported in four of the Dutch patients, as well as the English, Turkish, and Indian patient (sibling pairs were not identified). Heterozygosity was not detected in the remaining eight Dutch patients or the Arab patient, raising the possibility of a founder effect or identity by descent in these patients. In their manuscript, Lesnik Oberstein et al. 2006 note the presence of a CpG dinucleotide at the site of the mutation to provide a mechanism for the recurrence of this particular mutation. Our data as well as the B3GALTL sequences available in public databases (http://genome.ucsc.edu/) demonstrate that the sequence at the site of this mutation is CATGAGgtatgt and does not contain a CpG dinucleotide (Fig. 2). The second mutation reported by Lesnik Oberstein et al. 2006 encompasses a CpG dinucleotide (CCGCAgtacgtt) but this mutation does not represent a frequent recurrent mutation in the Peters Plus syndrome population. The mechanism of the recurrence of the c.660 + 1G > A mutation is unknown.
Our findings confirm that mutations in B3GALTL explain 100% of typical Peters Plus syndrome that is characterized by anterior chamber anomalies, characteristic facial features, short stature, and brachydactyly. Four patients with some but not all typical features of the Peters Plus phenotype were found to be B3GALTL mutation-negative in our study. Therefore the etiology of other conditions associated with Peters anomaly remains unknown.
There are multiple phenotypes associated with Peters anomaly reported in the literature. Peters anomaly has been linked to specific single gene defects as well as a variety of chromosomal anomalies [Maillette de Buy Wenniger-Prick and Hennekam, 2002]. Many patients with Peters anomaly demonstrate additional features that do not fit into known syndromes. Two reviews of patients with Peters anomaly without a diagnosed syndrome found additional structural systemic abnormalities in 42% [Heon et al., 1992] and 35% [Ozeki et al., 2000] of these cases. While these individuals did not have sufficient features to warrant a diagnosis of typical Peters Plus syndrome, many of the noted abnormalities are also seen in patients with Peters Plus syndrome, similar to the patients described here who were B3GALTL mutation-negative.
Regulatory elements of B3GALTL or genes in the B3GALTL pathway seem to represent likely candidate regions/genes to be involved in these phenotypes associated with Peters anomaly. The candidate factors include the family of TSR-containing proteins because the B3GALTL protein was found to interact with these sites. The TSR-containing protein family was shown to be involved in multiple biological processes such as regulation of cell adhesion, angiogenesis, cell proliferation and survival, protease function and cell- and axon-guidance. This family comprises such factors as thromborpondin-1, properdin, f-spondin, ADAMTS-13, members of the semaphorin 5 family and about 100 additional proteins [Adams and Tucker, 2000; Silverstein, 2002; Hess et al., 2008].
No carriers were identified in our screen of 360 chromosomes of control individuals similar to the results of Lesnik Oberstein et al. 2006, where 455 chromosomes of Dutch controls were screened with no carriers identified. These results suggest a very low carrier frequency for B3GALTL mutations, consistent with the rarity of Peters Plus syndrome.
Confirmation of the role of mutations in B3GALTL in Peters Plus syndrome is important for families of affected individuals. While prenatal ultrasound identified fetal anomalies in one previously reported patient [Boog et al., 2005], other affected patients have had normal prenatal ultrasounds [Frydman et al., 1991]. Within the population described here, nonspecific prenatal anomalies were detected in 50% of affected individuals. Identification of B3GALTL mutations in an affected individual will provide the family with the opportunity for prenatal molecular diagnosis in future pregnancies.
Acknowledgements
The authors thank the patients and their families for participation in our study. The authors are also grateful to Joseph Toonen for help with screening of select exons. This project was supported by awards EY013606 and EY015518 to EVS from the National Eye Institute at the National Institutes of Health (NIH), Children's Research Institute Foundation at Children's Hospital of Wisconsin grant, and General Clinical Research Center grant M01 RR00058 from the National Center for Research Resources, NIH.




