Genotype–phenotype relationship for localization and age distribution of telangiectases in hereditary hemorrhagic telangiectasia†
How to cite this article: Letteboer TGW, Mager H-J, Snijder RJ, Lindhout D, Ploos van Amstel H-K, Zanen P, Westermann KJJ. 2008. Genotype–phenotype relationship for localization and age distribution of telangiectases in hereditary hemorrhagic telangiectasia. Am J Med Genet Part A.
Abstract
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant disease characterized by arteriovenous malformations (AVMs) ranging from telangiectases to larger AVMs. Mutations in two genes cause HHT; ENG (HHT1) and ACVRL1 (HHT2). Although the hallmark for clinical diagnosis is the presence of telangiectases, there are few publications reporting the relative distribution and frequency of these features between HHT1 and HHT2. Here, the results of such analysis of telangiectases in 268 patients with HHT1 and 130 patients with HHT2 are described. Localization of the telangiectases is reported, and patients were clustered by age to estimate the site prevalence for different age categories. We show that telangiectases of the nasal mucosa are present at a higher prevalence and start to appear earlier in life than those of the oral mucosa or dermal sites in patients with either HHT1 or HHT2. Oral and nasal mucosal telangiectases are present earlier in life in patients with HHT1 compared to patients with HHT2, whereas dermal lesions are more frequent and appear earlier in life in patients with HHT2. In patients with either HHT1 or HHT2, the number of sites affected increases with age. In patients with HHT1, more women than men had skin telangiectases, particularly on the face. These results confirm that the frequency of AVMs differ between patients with HHT1 and HHT2, and that these differences can be detected on physical examination. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Hereditary hemorrhagic telangiectasia (HHT) or Rendu–Osler–Weber disease is inherited in an autosomal dominant pattern and is characterized by vascular dysplasia. Characteristic manifestations are epistaxis, telangiectases, and arteriovenous malformations (AVMs). Telangiectases are found predominantly on the face, lips, tongue and fingers and in the nasal, oral, and gastrointestinal mucosa. Larger AVMs occur mostly in the lungs (PAVM), brain (CAVM) and liver (HAVM) [Guttmacher et al., 1995].
Mutations in endoglin (ENG, chromosome 9q34, OMIM 131195) or in the activin receptor like kinase 1 gene (ACVRL-1, ALK1, chromosome 12q13, OMIM 601284) cause HHT1 and HHT2, respectively [McAllister et al., 1994; Johnson et al., 1996]. The ENG and ACVRL1 genes are predominantly expressed in vascular endothelium, and published data support haploinsufficiency as the main causative model of HHT. Mutations result in a reduction of the level of functional proteins and in dysregulation of the TGFβ pathway, which plays an important role in vascular homeostasis. It has been suggested that local processes (inflammation, cell injury) can act as trigger for the formation of AVMs and that modifier genes might play a crucial role in the development of the manifestations [Abdalla et al., 2003; Van den Driesche et al., 2003; Folz et al., 2004].
Although the same clinical manifestations occur in patients with HHT1 and HHT2, the frequency of the visceral manifestations vary [Bayrak-Toydemir et al., 2006; Bossler et al., 2006; Letteboer et al., 2006]. Furthermore, in HHT1 and HHT2 there is a considerable inter- and intra-familial variability for the visceral manifestations.
Little is known regarding the distribution and occurrence of telangiectases in patients with HHT1 and HHT2. In patients with HHT, telangiectases usually appear in the third decade of life and increase in size and in number [Plauchu et al., 1989; Shovlin and Letarte, 1999; Folz et al., 2004]. Plauchu et al. 1989 described the clinical manifestations in 324 patients with HHT and found telangiectases in 74% of the patients, with approximately half of the patients presenting before the age of 30 years. At that time it was not possible to distinguish between HHT1 and HHT2.
Berg et al. 2003 was the first to report on differences between patients with HHT1 or HHT2 regarding the frequency of the clinical manifestations. The results were based on 83 patients from the USA and the UK. Both epistaxis and telangiectases were found earlier in patients with HHT1 compared to patients with HHT2. Folz et al. 2004 described the telangiectases in 70 patients with HHT and found the highest frequency in the endonasal mucosa (88%) followed by the oral cavity (77%), facial skin (74%), and the hands (46%).
To date, no detailed age-related analysis has been performed and in most reports the age of appearance of the telangiectases is based on recollection of the patients. In this study we report on the presence and distribution of telangiectases in a large Dutch HHT cohort, based on the results of physical examination, performed by HHT specialists. This diminishes the inter-observer bias and avoids the problem of recall bias.
MATERIALS AND METHODS
The screening of family members of patients with HHT was performed from 1990 in the Dutch HHT center (St Antonius Hospital, Nieuwegein) [Haitjema et al., 1995]. Screening included an inspection for telangiectases on the first visit to the department. The skin (face, hands, legs, feet, thorax, abdomen, and neck), conjunctiva and oral mucosa (tongue, lip and mouth) were examined using a 2× magnifying lens. Each patient was examined by one of three senior clinicians (RS, JM, CW). The nose was inspected by an otorhinolaryngologist (FD) with an endoscope (magnification 10×). The sites of the telangiectases were scored on a standardized form. These forms were studied retrospectively in all Caucasian patients with a definite genetic diagnosis or clinical diagnosis according to the Curacao criteria [Shovlin et al., 2000]. In this study only Caucasian patients from families with ENG or ACVRL1 mutations were included, because patients of African descent have less visible telangiectases [Westermann et al., 2003].
Mutation analysis was performed to discriminate between HHT1 and HHT2 as described earlier [Letteboer et al., 2005]. When mutational analysis revealed a mutation in the proband, all clinically affected family members were considered to have the same mutation.
For each patient with HHT1 or HHT2, the location of telangiectases, the age at the time of examination, and the gender were scored. Dubious telangiectases were excluded. Telangiectases found using capillary microscopy of the nail fold were not included as telangiectases of the hands or arms, since this is not a widely used technique. Instead they were denoted as telangiectases “elsewhere.”
To gain insight into the relationship between telangiectases and age, patients were clustered into age groups, based on the age at the time of clinical investigation. The frequency of telangiectases in each group was calculated and studied in relation to HHT and gender. For each patient the number of sites involved was counted (including telangiectases elsewhere) and the average number of sites involved was calculated for each age group.
Statistical analysis was done using the chi square test. Bonferroni correction for multiple-comparison was not used because the comparisons were not considered independent tests. To correct for multiple testing a more stringent P-value was used, P values above 0.01 were considered to be statistically non-significant.
RESULTS
Of the 315 patients with HHT1, 47 were excluded and of the 131 patients with HHT2 one was excluded because of insufficient examinations of several sites, typically because the diagnosis was made because of a PAVM and a positive family history. The remaining 268 patients with HHT1 were from 56 families and 130 patients with HHT2 were from 39 families (Table I).
| HHT1, n | HHT2, n | |
|---|---|---|
| Patients investigated (M/F) | 315 (0.78) | 131 (0.66) |
| Excluded (M/F) | 47 (0.88) | 1 (F) |
| Mean age excluded (SD) | 45.2 years (22.3) | 73 years |
| Oral mucosa and skin (M/F) | 268 (0.76) | 130 (0.67) |
| Families | 56 | 39 |
| Males, (mean age; SD) | 116 (33.7 years; 17.8) | 52 (45.9 years; 15.7) |
| Females (mean age; SD) | 152 (36.5 years; 18.8) | 78 (42.5 years; 17.3) |
| Nasal mucosa (M/F) | 219 (0.82) | 108 (0.74) |
| Families | 53 | 36 |
| Males, (mean age; SD) | 99 (33.1 years; 18.2) | 46 (45.9 years; 15.9) |
| Females (mean age; SD) | 120 (36.0 years; 18.6) | 62 (42.4 years; 16.6) |
The age distribution shows that patients with HHT1 presenting with telangiectases were significantly younger than similar patients with HHT2. There was a non-significant female preponderance in both HHT1 (56.7%) and in HHT2 (60%). Men and women with HHT1 or HHT2 did not differ significantly regarding age (Table I).
The overall frequency of the HHT1 and HHT2 telangiectases at the various mucosal and dermal sites in both genders is shown in Table II. The nasal mucosa was only investigated in 219 patients with HHT1 and 108 with HHT2, because not all patients were seen by the otorhinolaryngologist. In the total group there was only a slight difference in the presence of nasal mucosal telangiectases; 95% in patients with HHT1 compared to 93% in patients with HHT2. Oral telangiectases (lip, tongue or mouth) were present in about 80% of all patients.
| HHT1 | HHT2 | |||||
|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | |
| n = 99 | n = 120 | n = 219 | n = 46 | n = 62 | n = 108 | |
| Nasal mucosa (%) | 92 (92.9) | 117 (97.5) | 209 (95.4) | 45 (97.8) | 55 (88.7) | 100 (92.6) |
| n = 116 | n = 152 | n = 268 | n = 52 | n = 78 | n = 130 | |
| Oral mucosa (%) | 88 (75.9) | 125 (82.2) | 213 (79.5) | 40 (76.9) | 65 (83.3) | 105 (80.8) |
| Tongue | 65 (56.0) | 108 (71.1) | 173 (64.6) | 33 (63.5) | 53 (67.9) | 86 (66.1) |
| Lip | 70 (60.3) | 101 (66.4) | 171 (63.8) | 32 (61.5) | 47 (60.3) | 79 (60.8) |
| Mouth | 36 (31.0) | 63 (41.4) | 99 (36.9) | 16 (30.8) | 27 (34.6) | 43 (33.1) |
| Conjunctivae (%) | 18 (15.5) | 18 (11.8) | 36 (13.4) | 10 (19.2) | 9 (11.5) | 19 (14.6) |
| Dermal total (%) | 55 (47.4) | 103 (67.8) | 158 (59) | 39 (75.0) | 57 (73.1) | 96 (73.8) |
| Hands, arms | 36 (31.0) | 85 (55.9) | 121 (45.1) | 36 (69.2) | 45 (57.5) | 81 (62.3) |
| Face | 34 (29.3) | 43 (28.3) | 77 (28.7) | 22 (42.3) | 30 (38.5) | 52 (40.0) |
| Chest | 5 (4.3) | 16 (10.5) | 21 (7.8) | 8 (15.4) | 7 (9.0) | 15 (11.5) |
| Neck | 9 (7.8) | 14 (9.2) | 23 (8.6) | 6 (11.5) | 2 (2.6) | 8 (6.1) |
| Feet, legs | 3 (2.6) | 1 (0.7) | 4 (1.5) | 2 (3.8) | 1 (1.3) | 3 (2.3) |
| Abdomen | 0 (0) | 1 (0.7) | 1 (0.4) | 0 (0) | 0 | 0 (0) |
- The number of patients is depicted for each group (n). The numbers represent the number of patients in which the telangiectases for the particular site were detected. Between brackets the percentage of patient affected for the site.
The prevalence of dermal telangiectases is lower than the prevalence of nasal and oral lesions, in patients with either HHT1 or HHT2. Dermal lesions are found more often in patients with HHT2 (74%) compared to HHT1 (59%; P = 0.004), most significantly when comparing telangiectases on the hands (62% and 45%; P = 0.001), but also a suggestive difference in facial telangiectases (40% and 29%; P = 0.02).
The proportion of patients with conjunctiva involvement is similar for both HHT1 (13%) and HHT2 (15%). Telangiectases on the neck, feet and abdomen are found only in a minority of patients with HHT. The low prevalence on the feet compared to the hands has been observed before [Plauchu et al., 1989; Folz et al., 2004]. Significant differences between men and women were only found for dermal telangiectases in HHT1 with a higher prevalence in women (47% and 68%; P = 0.0008), particularly for the hands (31% and 56%; P = 4.9 × 10−5).
The presence of telangiectases in the different age groups on the various mucosal and dermal sites for HHT1 and HHT2 are depicted in Table III and Figures 1 and 2. The frequency increases with age in both forms for all sites except the neck, feet and abdomen, which are rarely affected. A steeper curve was found in nasal and oral mucosa compared to the skin, indicating that mucosal lesions tend to appear earlier and in a higher proportion of the patients.
| HHT1 | HHT2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71+ | 0–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71+ | |
| Number (M/F) | n = 20 (1.2) | n = 42 (0.75) | n = 36 (1.38) | n = 32 (0.39) | n = 41 (0.78) | n = 30 (0.76) | n = 12 (1.0) | n = 6 (0.20) | n = 5 (0.67) | n = 4 (0.33) | n = 11 (0.83) | n = 23 (0.44) | n = 28 (0.87) | n = 19 (1.11) | n = 12 (0.71) | n = 6 (1.0) |
| Mean age (years) | 5.9 | 15.9 | 25.6 | 35.4 | 45.6 | 55.2 | 65.5 | 74.0 | 7.6 | 15.5 | 26.4 | 36.1 | 44.6 | 55.1 | 64.7 | 74.0 |
| Nasal mucosa (%) | 16 (80) | 40 (95.2) | 34 (94.4) | 32 (100) | 41 (100) | 29 (96.7) | 11 (91.7) | 6 (100) | 4 (80.0) | 2 (50) | 7 (63.6) | 23 (100) | 27 (96.4) | 19 (100) | 12 (100) | 6 (100) |
| Number (M/F) | n = 24 (1.0) | n = 47 (0.68) | n = 45 (1.5) | n = 40 (0.54) | n = 51 (0.70) | n = 38 (0.65) | n = 16 (0.78) | n = 7 (0.17) | n = 5 (0.67) | n = 8 (0.14) | n = 14 (0.75) | n = 25 (0.47) | n = 33 (0.83) | n = 22 (1.0) | n = 16 (0.60) | n = 7 (0.75) |
| Mean age (years) | 6.0 | 15.9 | 25.8 | 35.4 | 45.2 | 55.4 | 65.8 | 74.3 | 7.6 | 16.5 | 26.2 | 36.1 | 44.9 | 55.3 | 65.0 | 74.1 |
| Oral mucosa (%) | 4 (16.7) | 25 (53.2) | 39 (86.7) | 36 (90.0) | 50 (98.0) | 37 (97.4) | 15 (93.8) | 7 (100) | 2 (40.0) | 2 (25.0) | 8 (57.1) | 22 (88.0) | 28 (84.8) | 21 (95.5) | 16 (100) | 6 (85.7) |
| Tongue | 2 (8.3) | 16 (34.0) | 28 (62.2) | 28 (70.0) | 42 (82.4) | 36 (94.7) | 14 (87.5) | 7 (100) | 1 (20.0) | 1 (12.8) | 5 (35.7) | 18 (72.0) | 22 (66.7) | 18 (81.8) | 15 (93.8) | 6 (85.7) |
| Lip | 4 (16.7) | 15 (31.9) | 30 (66.7) | 30 (75.0) | 39 (76.5) | 33 (86.8) | 13 (81.3) | 7 (100) | 0 (0) | 1 (12.5) | 4 (28.6) | 17 (68.0) | 22 (66.7) | 16 (72.7) | 15 (93.8) | 4 (57.1) |
| Mouth | 0 (0) | 6 (12.8) | 12 (26.7) | 16 (40.0) | 29 (56.9) | 23 (60.5) | 8 (50.0) | 5 (71.4) | 1 (20.0) | 0 (0) | 1 (7.1) | 8 (32.0) | 11 (33.3) | 10 (45.5) | 9 (56.3) | 3 (42.9) |
| Conjunctivae (%) | 0 (0) | 3 (6.4) | 2 (4.4) | 8 (20.0) | 8 (15.7) | 10 (26.3) | 3 (18.8) | 2 (28.6) | 0 (0) | 0 (0) | 2 (14.3) | 3 (12.0) | 2 (6.1) | 6 (27.3) | 4 (25.0) | 2 (28.6) |
| Dermal total (%) | 7 (29.2) | 16 (34.0) | 18 (40.0) | 27 (67.5) | 39 (76.5) | 32 (84.2) | 13 (81.3) | 6 (85.7) | 3 (60.0) | 2 (25.0) | 6 (42.9) | 17 (68.0) | 27 (81.8) | 21 (95.5) | 14 (87.5) | 6 (85.7) |
| Hands, arms | 3 (12.5) | 14 (29.8) | 13 (28.9) | 20 (50.0) | 30 (58.8) | 26 (68.4) | 12 (75.0) | 3 (42.9) | 2 (40.0) | 1 (12.5) | 6 (42.9) | 14 (56.0) | 22 (66.7) | 17 (77.3) | 13 (81.3) | 6 (85.7) |
| Face | 4 (16.7) | 1 (2.1) | 3 (6.7) | 11 (27.5) | 20 (39.2) | 24 (63.2) | 9 (56.3) | 5 (71.4) | 1 (20.0) | 2 (25.0) | 1 (7.1) | 7 (28.0) | 12 (36.4) | 17 (77.3) | 7 (43.8) | 5 (71.4) |
| Chest | 0 (0) | 2 (4.3) | 3 (6.7) | 1 (2.5) | 7 (13.7) | 6 (15.8) | 1 (6.3) | 1 (14.3) | 0 (0) | 0 (0) | 1 (7.1) | 4 (16.0) | 7 (21.2) | 1 (4.5) | 0 (0) | 2 (28.6) |
| Neck | 2 (8.3) | 1 (2.1) | 6 (13.3) | 4 (10.0) | 4 (7.8) | 4 (10.5) | 2 (12.5) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) | 3 (9.1) | 2 (9.1) | 0 (0) | 2 (28.6) |
| Feet, legs | 0 (0) | 0 (0) | 2 (4.4) | 0 (0) | 2 (3.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6.1) | 1 (4.5) | 0 (0) | 0 (0) |
| Abdomen | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mean localizations | 1.7 | 2.6 | 3.3 | 4.0 | 4.5 | 5.3 | 4.7 | 5.1 | 2 | 1.3 | 2.4 | 4.1 | 4.2 | 5.1 | 4.8 | 5.6 |
- The presence of telangiectases per age group is given in numbers (n) and the percentage (between brackets) is the number of patient with telangiectases divided by the total of the corresponding age group (n).
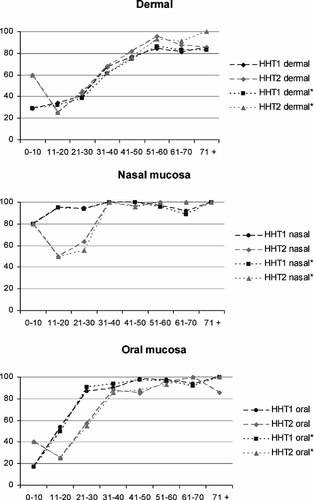
Graphical view of the dermal (hands/arms, chest, feet/legs, and abdomen), oral (tongue, lip, and mouth) and nasal telangiectases. Age groups (years) are depicted on the X-axis, proportion of the patients with telangiectases for the site on the Y-axis. The different point prevalence's are connected. In an attempt to correct for possible referral bias, HHT* shows the results when the probands of the families were excluded.
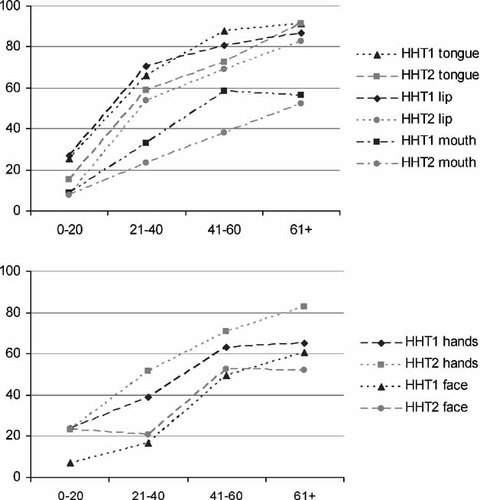
Graph of separate oral localizations (lip, tongue mouth) and dermal localizations (hands and face) per age group. Age groups (years) are depicted on the X-axis, proportion of the patients with telangiectases for the particular site on the Y-axis. The different point prevalence's are connected.
Nasal telangiectases are present at a younger age in patients with HHT1 compared to those with HHT2. Over 90% of the patients with HHT1 have nasal lesions in the age group 0–20 in HHT1, compared to 67% in the HHT2 group 0–20. Also, oral mucosal lesions in general occur at a younger age in patients with HHT1. In the age group 21–30 87% of the patients with HHT1 have oral lesions, whereas 88% of the patients with HHT2 have oral involvement 10 years later, in the age group 31–40. Among the individual sites (tongue, lip, mouth) the difference is most striking on the lip.
In contrast, dermal telangiectases in general tend to be present at a younger age in patients with HHT2 than in patients with HHT1. The individual dermal sites also do not show a significant difference in the age-related prevalence between HHT1 and HHT2. The point prevalence of telangiectases in the conjunctivae increases with age, but there is no difference between HHT1 and HHT2.
The mean number of sites with telangiectases also increases with increasing age (Table III). More sites were involved at a younger age in patients with HHT1 than in those with HHT2.
To correct for possible referral bias, the analyses per age group was also performed excluding the probands, the first patient of each family to attend the HHT center. The results of this analysis show that excluding the probands resulted in negligible changes in the results (Fig. 1).
In both, the HHT1 and HHT2 groups, there were eight patients without telangiectases found on physical examination. In the HHT1 group, six of the eight patients were ≤10 years. Two of the six patients had a PAVM and three of the six had regular nosebleeds. Only two of the six patients had no epistaxis, no telangiectases and no visceral manifestations, but were diagnosed using DNA analysis. The two remaining patients with HHT1 (23 years and 53 years) had regular nosebleeds, but no other HHT manifestations.
Among patients with HHT2, three (7, 22, and 34 years) had no telangiectases or other HHT symptoms. One patient without telangiectases (27 years) had epistaxis, a PAVM, and a HAVM. Four patients (12, 20, 29, and 37 years) had nosebleeds regularly, without other manifestations of HHT.
DISCUSSION
In this study, the sites of telangiectases on skin and mucosa were scored. The number of lesions per site was not considered, because telangiectases may be numerous and counting may be difficult and subject to observer bias. Moreover, the number does not add to diagnostic accuracy, as long as the typical telangiectases are present. The results of this analysis give a cross sectional view of the frequency and distribution of telangiectases in patients with HHT. Telangiectases were studied in families with confirmed HHT1 or HHT2 mutations.
We observed that patients with HHT1 visited the outpatient department at a younger age than did patients with HHT2. This difference persisted after exclusion of the probands. This is not surprising, since more patients with HHT1 have PAVMs and CAVMs [Bayrak-Toydemir et al., 2006; Bossler et al., 2006; Letteboer et al., 2006], and most probands are initially referred because of visceral manifestations, mostly PAVMs. This will bias towards screening of family members of patients with HHT1 at a younger age, as compared to those with HHT2. To circumvent this problem, analysis by age group was performed.
A bias in the data for telangiectases is therefore not likely, but cannot be excluded. If the pathogenesis of the visceral manifestations and the telangiectases are overlapping, then referral with a PAVM might introduce bias. To correct for this, stratification by age was also performed discarding the initially referred proband of the family (Fig. 1). The graphs show only a slight change, indicating that the probands did not cause a major distortion in the analysis. In our HHT2 population, in the youngest group (0–10) more patients had telangiectases than in the next older group (11–20). Although the youngest group is small (n = 5), the higher frequency might reflect an ascertainment bias. Parents who suspect telangiectases in their children at a younger age might attend the HHT center earlier. Another potential bias is the exclusion of 47 patients with HHT1, but only one with HHT2. These patients were referred for treatment of a PAVM and were therefore not thoroughly examined for telangiectases. Exclusion of probands (also referred with PAVM) did not change the results of the analysis; we conclude that the results did not change significantly because of exclusion of these patients.
The HHT phenotype has an age-dependent penetrance. The analysis by age group is therefore important and useful for clinicians. Age-dependent penetrance of telangiectases has been reported [Plauchu et al., 1989; Berg et al., 2003; Folz et al., 2004], based on the recollection of the patients. To study the relationship of age and penetrance of telangiectases, we performed physical examination. We show that with increasing age the frequency of telangiectases increases, for the nasal mucosa, the oral mucosa (tongue, lip, mouth), the conjunctiva and the skin of the hands and face. Furthermore, the nasal mucosa was affected significantly earlier in patients with HHT1 compared to those with HHT2. This confirms the findings of Berg et al. 2003, who showed an earlier onset of epistaxis in the patient with HHT1. A high prevalence of telangiectases in the nasal mucosa in patients below the age of 21 in those with HHT1 (90%) or HHT2 (67%) was found. In contrast, oral and dermal lesions are at this age present in a minority of the patients. This indicates that nasal endoscopy is a useful tool to establish a clinical diagnosis.
When comparing our results to earlier publications, we encountered three major problems. First, since there is an age-dependent penetrance for the symptoms, including telangiectases, the age of the population is crucial. In the reports by Plauchu et al. 1989 and Folz et al. 2004 the (mean) age of the investigated population is not specified. In both reports the population appeared to be younger, which explains the lower frequencies of telangiectases in those reports. Second, Plauchu et al. 1989 and Folz et al. 2004 report on the frequency of telangiectases in patients with HHT, comprising a mixture of patients with ENG mutations, ACVRL1 mutations, and possibly other genes. Subsequent genetic analysis revealed that ACVRL1 mutations are found more often in France than ENG mutations, whereas in the Dutch population ENG mutations are more frequent [Lesca et al., 2004; Letteboer et al., 2005]. This apparent population difference between the French and the Dutch HHT populations can also explain part of the observed differences. Third, there is the problem of ascertainment bias. The way the patients were ascertained indicates what kind of referral bias one has to anticipate and how to correct for it. The difference in telangiectases in the nose is striking. The nose was affected in the French population in 37% of the patients whereas epistaxis was present in 96% of the patients. Our panel showed nasal telangiectases in 92% of the patients with HHT2 and in 95% of those with HHT1. Since epistaxis in patients with HHT is usually caused by nasal telangiectases, a prevalence of nasal telangiectases of 37% seems surprisingly low. The strong concordance between epistaxis and nasal telangiectases in the Dutch population might be explained by the endoscopic examination used here.
In HHT1 more patients develop a PAVM and/or a CAVM compared to HHT2 [Bayrak-Toydemir et al., 2006; Bossler et al., 2006; Letteboer et al., 2006]. HHT1 has also an earlier onset of telangiectases of the nasal and oral mucosa. The mechanisms leading to the inter- and intrafamilial variability in HHT are not obvious. Most likely these differences are caused, by a combination of multiple modifier genes interacting with several (maybe tissue specific) environmental factors in a complex manner. An important role for environmental factors in dermal telangiectases, is very likely, since the sun exposed skin (face and hands) are most often affected. In a normal population, telangiectases were associated with sun exposure, smoking, and increasing age [Kennedy et al., 2003]. Although these telangiectases are distinct from the telangiectases found in HHT, the association found in the healthy population might also hold true for the HHT population. The high frequency of telangiectases in the nasal and oral mucosa might be a reflection of the density and superficial localization of arteries in mucosal tissue in general. It is more likely that the high frequency is explained by a combination of the density and environmental factors; possible environmental factors being local stress, infection, minor trauma, exposure to toxic agents, and temperature changes.
Gender-associated differences in patients with HHT1 and HHT2 have been reported for the visceral manifestations [Letteboer et al., 2006]. The observed higher frequency of dermal telangiectases in women, significantly in those with HHT1, particularly on the hands, is in concordance with that observation. If this sex difference is confirmed, it will be worthwhile to devote research to its causal factors and mechanisms.
In conclusion, we show that in patients with HHT1 and patients with HHT2, telangiectases are identified in descending order in the nasal mucosa, oral mucosa, and skin. The number of affected sites increase with increasing age, in both HHT1 and HHT2. In general, oral and nasal lesions tend to appear earlier in patients with HHT1, whereas dermal lesions are observed earlier in patients with HHT2. This predilection of sites is likely to be the result of a combination of multiple modifier genes interacting with several (maybe tissue specific) environmental factors in a complex manner.
Acknowledgements
We thank Rosemary Akhurst for reviewing the article.




