A novel recurrent mitochondrial DNA mutation in ND3 gene is associated with isolated complex I deficiency causing Leigh syndrome and dystonia†
How to cite this article: Sarzi E, Brown MD, Lebon S, Chretien D, Munnich A, Rotig A, Procaccio V. 2007. A novel recurrent mitochondrial DNA mutation in ND3 gene is associated with isolated complex I deficiency causing Leigh syndrome and dystonia. Am J Med Genet Part A 143A:33–41.
Abstract
Defects in NADH:ubiquinone oxidoreductase (complex I), the largest complex of the mitochondrial respiratory chain, account for most cases of respiratory chain deficiency in human. Complex I contains at least 45 subunits, 7 of which are encoded by mitochondrial DNA (mtDNA). Here we report a novel 10197G>A mutation of the ND3 gene in three unrelated families with Leigh syndrome (LS) or dystonia. Variable degrees of heteroplasmy were found in all tissues tested and a high percentage of mutant mtDNA was observed in muscle. The 10197G>A mutation modifies a hydrophobic alanine residue into a hydrophilic threonine (A47T) in a highly conserved domain of ND3 subunit. Furthermore, this defect could be transferred along with the mutant mtDNAs to ρ° lymphoblastoid cells in cybrid experiments. However, nuclear modifier genes may also play a role in the phenotypic expression and severity of the 10197G>A mutation. The association of the 10197G>A ND3 mutation with an isolated biochemical defect involving complex I and the discovery of the 10197G>A mutation with a similar phenotype in three unrelated families establish its pathogenicity and demonstrate that the amino acid position A47 is important for the function of complex I. These results show that the 10197G>A mutation in the mitochondrial ND3 gene should be considered as a common mtDNA mutation responsible for LS and dystonia. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Complex I or NADH-ubiquinone oxidoreductase (EC 1.6.5.3) is the first component of the mitochondrial respiratory chain. This enzyme oxidizes NADH to reduce ubiquinone and is coupled with proton translocation across the mitochondrial inner membrane. Complex I, the largest complex of the respiratory chain, is composed of at least 45 subunits with only 7 (ND1-ND6, ND4L) encoded by the mitochondrial genome [Hirst et al., 2003]. Complex I accounts for most cases of respiratory chain deficiency in human and results in a wide range of clinical presentations, including Leigh syndrome (LS, MIM 256000) or dystonia (MIM 128100) [von Kleist-Retzow et al., 1998; Loeffen et al., 2001]. In most cases, the family history is consistent with sporadic or autosomal recessive inheritance. However, previous studies have shown that at least 20% of respiratory chain complex I deficiencies are related to heteroplasmic mitochondrial DNA (mtDNA) mutations [Kirby et al., 2004a]. Some mtDNA mutations occur de novo [Lebon et al., 2003; Crimi et al., 2004; McFarland et al., 2004] but other are maternally inherited with healthy mothers harboring low level of mutant mtDNA [Taylor et al., 2001; Chol et al., 2003; Leshinsky-Silver et al., 2005].
In general, complex I dysfunction due to either mtDNA or nuclear DNA (nDNA) mutations are relatively common in LS, one of the most common and severe mitochondrial neurodegenerative disorders [see http://www.mitomap.org/ and Wallace et al., 2006]. mtDNA mutations have also been linked to movement disorders such as dystonia. For instance, a mutation at position 14459G>A in the complex I ND6 subunit was identified in several families with Leber's hereditary optic neuropathy and dystonia [Shoffner et al., 1995]. We report here three independent families with a 10197G>A mutation in the mitochondrial ND3 gene. All children are affected with severe LS and isolated complex I deficiency. Mothers of two of these three families are moderately affected but the third mother is healthy. We also show that the severity of the disease is related to the mutant load in various tissues of the patients but may also result to the influence of additional modifier genes. Cybrid cells resulting from fusion of patient lymphoblasts and ρ° cells still maintain a complex I deficiency demonstrating the pathological nature of the mutation. These results show that the 10197G>A mutation in the mitochondrial ND3 gene should be considered as a common mtDNA mutation responsible for LS and dystonia.
MATERIALS AND METHODS
Patients
Patient 1, the mother of family 1 (Fig. 1), is of French origin. She is now 41 and presented with hypotonia and a mild mental retardation at the age of 14. Plasma lactate and lactate/pyruvate molar ratios were normal. Patient 2, her first boy was born after normal pregnancy and term delivery (birth weight 2.8 kg, height 47 cm, head circumference 33 cm). Growth retardation as well as hypotonia and psychomotor regression appeared at 1 month of age. He rapidly presented seizures and a CT scan showed hypodensity of the brainstem suggestive of LS. Elevated cerebrospinal fluid (CSF) lactate was noted (3.5 mmol/L). He died at 5 months of age. The second boy (Patient 3) presented at 2 months of age with anorexia, nystagmus, hypotonia, elevated plasma lactate (4.27 mmol/L, N < 2.8) and lactate/pyruvate molar ratio (37, N < 20). A CT scan showed hypodensity of lentiform nuclei. He rapidly died at 2 months of age. The third child (Patient 4), a girl (birth weight 2.74 kg, height 45 cm, head circumference 32 cm) was normal during her first month of life. She then presented with hypotonia and growth retardation. She had liver enlargement and slight muscle atrophy. Mildly elevated plasma lactate was noted (2.8 mmol/L). She died at 8 months of age of respiratory distress.
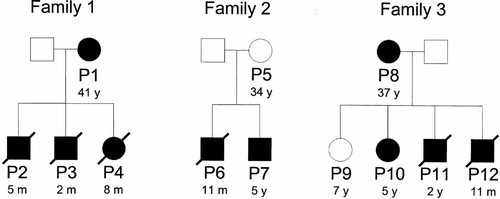
Pedigrees of the three unrelated complex I-deficient families. Solid symbols indicate affected individuals and open symbols represent unaffected individuals. P1 to P12: Patients 1 to 12. Ages of patients or at the time of death are indicated (y: years; m: months).
The mother of family 2 of French origin (Patient 5) is now 34 years old and is healthy (Fig. 1). Her first boy (Patient 6) was born after normal pregnancy and delivery. He presented with trunk hypotonia, pyramidal syndrome, and psychomotor retardation at 4 months of age. High plasma lactate (4.6 mmol/L) with elevated lactate/pyruvate molar ratio (25) and CSF lactate were noted (2.1 mmol/L). At 6 months of age, MRI revealed bilateral hyperdensities in lentiform nuclei, thalamus and red nuclei suggesting LS. One month later, he developed myoclonic epilepsy and died at 11 months of age. His young brother (Patient 7) was normal for gestational age (birth weight 3.45 kg, height 48 cm, head circumference 36 cm). He presented with trunk hypotonia, peripheral hypertonia, and plagiocephalia at 5 months of age. Plasma lactate was normal but CSF lactate was elevated (2.35 mmol/L). An MRI revealed hypodensity of basal ganglia related to LS. He thrived normally but at 2 years of age a severe psychomotor delay was noted. The boy is now 5 years old and has strabismus, epilepsy, dystonia, and pyramidal syndrome.
Patient 8, the mother of family 3 (Fig. 1), is 37 years old. Birth and development were unremarkable. At age 6 years she developed a generalized dystonia affecting predominantly her extremities and oral-facial movements associated with bilateral basal ganglia lesions. Histology and electron microscopy performed on her skeletal muscle were unremarkable. She had four children, a 7-year-old unaffected daughter (Patient 9) and three affected children. The second, a 5-year-old girl (Patient 10), is affected with seizures and developmental delay since age 5 months. The third child (Patient 11) presented with severe progressive encephalopathy and seizures leading to death at 2 years of age. Plasma and urinary lactate and pyruvate were constantly normal. Patient 12, a 9-month-old boy, was born at term after an uncomplicated pregnancy and delivery. He did well until age 5 months when he was examined because of delay in motor milestones and hypotonia. Plasma and urinary lactate and pyruvate were normal. At that age, he developed seizures and brain MRI showed involvement of basal ganglia, thalamic and brain stems lesions consistent with LS and died at 11 months of age. Histological and electron microscopy analyses performed on skeletal muscle did not reveal any specific changes. The parents of the three families are nonconsanguineous.
Cell Culture
Skin fibroblasts were grown in RPMI 1640 medium supplemented with glutamax (446 mg/L), 10% fetal bovine serum (FBS), 100 µg/ml streptomycin, 100 IU/ml penicillin, 200 µmol/L uridine, and 2.5 mmol/L sodium pyruvate at 37°C under standard conditions. Lymphoblastoid cell lines were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) with high glucose (4.5 mg/ml) supplemented with 10% FBS and 50 µg/ml uridine and 1 mM sodium pyruvate. Lymphoblastoid cell lines from patients and controls were then enucleated and fused with Wal2a ρ° cells to generate cybrid cells as described [Trounce et al., 1994]. The resulting cybrid cells were subsequently expanded and biochemical assays were performed on isolated mitochondria.
mtDNA Analysis
Genomic DNA was extracted from skeletal muscle, blood leukocytes, skin fibroblasts or liver by standard methods. mtDNA was screened in skeletal muscle for mitochondrial rearrangements by Southern blot and the mitochondrial genome of all patients was screened for the most common known mutations at position 3243A>G, 3271T>C, 8344A>G, 8356T>C, 8993T>G or T>C, and 14459G>A. Mitochondrial genes encoding subunits of complex I (ND1-ND6 and ND4L) were submitted to PCR amplification after an initial denaturation at 96°C for 5 min, followed by 20 cycles of 96°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, and a last extension at 72°C for 10 min. A list of oligonucleotide primers used for mtDNA amplification and sequencing is available upon request. PCR products were purified by ExoSAP-IT® (GE Healthcare, Piscataway, NJ) and directly sequenced using the PRISM™ Ready Reaction Sequencing Kit (PE Applied Biosystems, Foster City, CA) on an automatic sequencer (ABI 3130, PE Applied Biosystems). Sequence data were analyzed using Sequencher (version 4.0.5, Genecode Corp., Ann Arbor, MI) software.
Quantification of Heteroplasmy
The 10197G>A mutation eliminates a Cac8I-specific restriction site which was used for Restriction Fragment Length Polymorphism (RFLP) analysis in family 3. A 244-bp fragment was PCR amplified using the primers 5′-AGAGTAATAAACTTCGCCTTA-3′ (nt 10053–10073) and 5′-GTAGGGCTCATGGTAGGG-3′ (nt 10296–10279) and subsequently digested with Cac8I. The forward primer was FAM-tagged and the fluorescent fragments were analyzed on an automatic sequencer (ABI 3130, PE Applied Biosystems) using the Genescan and Genotyper softwares (Applied Biosystems) [Gigarel et al., 2005]. The mutant load was quantified as detailed in Figure 2 legend.
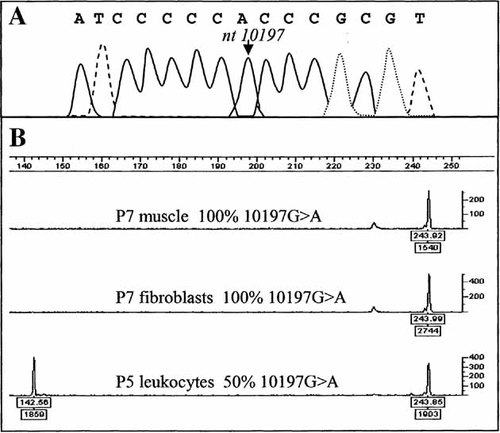
Analysis of the 10197G>A mutation of the ND3 gene. A: Sequence analysis of ND3 gene in Patient 7. The arrow shows the mutation. B: Determination of the 10197G>A mutant load in patients P7 (muscle and fibroblast cells) with 100% mutant load in both tissues and P5 (leukocytes) with 50% mutant. The 10197G>A mutation abolishes a Cac8I restriction site. The Cac8I restriction digest of the 244-bp PCR product generated a 148-bp + 96-bp fragments and a 244-bp fragment for wild-type and mutant species, respectively. After electrophoresis using an ABI3130 genetic analyzer (Applied Biosystems), fluorescent fragments (244-bp and 148-bp) were detected using Genescan and Genotyper softwares. Peak areas are indicated under the base line. Mutant load was calculated by dividing the 244-bp peak area by the sum of the 244-bp and 148-bp peak areas.
Mitochondrial Isolation and OXPHOS Enzymology
Mitochondrial isolation, polarographic and spectrophotometric studies of respiratory chain enzymes were carried out on skeletal muscle, liver homogenate and/or skin fibroblasts, lymphoblastoid cell lines, and cybrids as previously described [Rustin et al., 1994; Trounce et al., 1996]. Mitochondrial protein determination was measured using the Lowry method. Spectrophotometric analysis of the respiratory chain complexes was performed in mitochondrial preparations from muscle using a dual beam UV-visual spectrophotometer (model DW-2000, SLM-Aminco, Urbana, IL). All experiments were done at least in triplicates.
RESULTS
The biochemical assays of mitochondrial respiratory chain activities in tissues and cultured cells revealed a significant reduction of complex I and complex I-linked activities in all patients studied. Interestingly, there was a tissue-specific difference in the magnitude of mitochondrial biochemical defect. For example, in muscle homogenate of Patient 2, all respiratory chain activities were decreased (Table I). However, in lymphoblast and muscle mitochondria of Patients 8 and 12, a severe isolated complex I deficiency was identified (Tables I and II) and in liver homogenate, complex I activity was in the lower normal values (Table I). In general, however, a complex I defect is the most pronounced and consistent deficiency found in the mitochondrial respiratory chain enzymes.
| Activity (nmol/min/mg protein) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enzymes | Muscle homogenate | Muscle mitochondria | Liver homogenate | Fibroblasts | ||||||
| P2 | Controls (n = 200)a | P7 | P8 | P12 | Controls (n = 50)a | P7 | Controls (n = 150)a | P7 | Controls (n = 200)a | |
| I | 1.5 | 17.5 ± 4.7 | ND | 0 | 6 | 106 ± 46 | 16 | 22.4 ± 4.6 | 25 | 35.2 ± 9.4 |
| II | 19 | 31.8 ± 7.7 | — | — | — | — | 174 | 139.2 ± 18.4 | 78 | 256 ± 32.7 |
| III | 106 | 346.1 ± 75.1 | 471 | 415 | 876 | 1389 ± 368 | 260 | 358.1 ± 57.6 | 704 | 718 ± 185 |
| IV | 78 | 151.6 ± 37.7 | 406 | 867 | 642 | 1210 ± 353 | 325 | 183.5 ± 30.0 | 593 | 352.5 ± 86.9 |
| I + III | 8.7 | 26.5 ± 9.8 | 87 | 7 | 64 | 262 ± 93 | — | — | — | |
| II + III | 16 | 26.2 ± 7.9 | 123 | 398 | 258 | 526 ± 140 | 95 | 68.1 ± 12.9 | 188 | 256.2 ± 32.7 |
- Abnormal values are in bold. Complex I, II, III, IV, I + III, II + III: activities of the various respiratory chain complexes (expressed as nmol/min/mg protein).
- a Control values are mean ± standard deviation (SD).
| Activities (nmol/min/mg protein) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Enzymes | Lymphoblastsa | Wal2A Cybridsb | ||||||
| P8 | P9 | P12 | Controlsc | P8 | P9 | P12 | Controlsc | |
| I | 20 | 93 | 16 | 49 ± 17 | 21 | 14 | 8 | 43 ± 14 |
| III | 397 | 771 | 310 | 472 ± 113 | 505 | 248 | 434 | 691 ± 224 |
| IV | 557 | 1091 | 737 | 548 ± 193 | 412 | 463 | 910 | 436 ± 78 |
- Complex I, III, IV: activities of complexes I, III and IV (expressed as nmol/min/mg protein). Abnormal values are in bold.
- a 74%, 73%, and 100% mutant mtDNA for P8, P9, and P12, respectively.
- b P8, P9 and P12 cybrids contained 97%, 69%, and 100% mutant mtDNA, respectively.
- c Control values are mean ± SD.
Based on mitochondrial enzyme activities, PCR amplification and sequence analysis of all mitochondrial complex I genes were performed. A homoplasmic G-to-A transition at nucleotide 10197 of the ND3 gene was detected in muscle of Patients 2, 4, 7, 8, and 12 (Fig. 2 and Table III). This substitution was subsequently found in additional family members after PCR amplification and direct sequencing of the ND3 gene; and the presence of the mutation was confirmed by fluorescent PCR and RFLP analysis using the Cac8I restriction assay in the three families. A high level of mutant mtDNA was detected in several tissues of the various patients. Indeed, homoplasmy for the mutant mtDNA species was observed in muscle of Patients 2, 4, 7, and 12 as well as in liver of Patient 3 and fibroblasts of Patient 7, Patient 8 was heteroplasmic in skeletal muscle with a mutant load of 96% (Fig. 2 and Table III). Leukocytes of the two affected mothers (Patients 1 and 8) presented 67% and 74% of 10197G>A mutation, respectively, while the healthy mother (Patient 5) of Patients 6 and 7 showed only 50% of mutant load (Fig. 2 and Table III). The mtDNA from skeletal muscle was fully sequenced in Patient 12 and no other pathogenic mutations were identified as all other base substitutions represented previously reported mtDNA polymorphisms in the Mitomap database [Brandon et al., 2005]. In addition, we screened the entire mitochondrial genome using surveyor nuclease as previously described [Bannwarth et al., 2005] that can detect mtDNA mutants present at as low as 3% and no other heteroplasmic mtDNA mutations were found.
| Family | Patient | Clinical Status | Tissue | Mutant mtDNA (%) |
|---|---|---|---|---|
| 1 | P1 | Affected | Leukocytes | 67 |
| P2 | Affected | Skeletal muscle | 100 | |
| P3 | Affected | Liver | 100 | |
| P3 | Affected | Leukocytes | 100 | |
| P4 | Affected | Skeletal muscle | 100 | |
| 2 | P5 | Unaffected | Leukocytes | 50 |
| P7 | Affected | Skeletal muscle | 100 | |
| P7 | Affected | Fibroblasts | 100 | |
| 3 | P8 | Affected | Skeletal muscle | 96 |
| P8 | Affected | Leukocytes | 74 | |
| P9 | Unaffected | Leukocytes | 73 | |
| P10 | Affected | Leukocytes | 100 | |
| P12 | Affected | Skeletal muscle | 100 | |
| P12 | Affected | Leukocytes | 100 | |
| P8 | Cybrids | 97 | ||
| P9 | Cybrids | 69 | ||
| P12 | Cybrids | 100 |
- Mutant loads in muscle, leukocytes, liver or fibroblasts of the various patients.
We determined the mtDNA haplotype for our three families. Families 1, 2, and 3 belong to independent mitochondrial Caucasian haplogroups U5, H, and V, respectively. The Cac8I RFLP assay provided a rapid test for the population frequency of this mutation which was not be found in a series of more than 230 Caucasian control individuals which have no history of neurological or metabolic syndromes. In addition, maternally related family members of all three families were screened for the presence of the 10197G>A mutation using fluorescent PCR quantification. Two family members, the more mildly affected mother (Patient 8) and the sister (Patient 9) exhibited heteroplasmy at the 10197 position in leukocytes with 74% and 73% mutant loads, respectively. The proband (Patient 12) and his affected sister (Patient 10) were homoplasmic for the mutation in leukocytes, showing a correlation between the mutant load and severity of neurological symptoms (Fig. 2 and Table III).
The 10197G>A mutation changes a hydrophobic alanine residue into a hydrophilic threonine (A47T) in a highly conserved part of the ND3 subunit (Fig. 3). The analysis of the predicted structural model of ND3 was performed using TMPRED program (http://www.ch.embnet.org/software/TMPRED_form.html) predicting three putative transmembrane helices (Fig. 4). This evolutionary conserved amino acid substitution is located in an extramembrane loop facing the mitochondrial matrix next to two other pathogenic mutations at positions 10158T>C (S34P) and 10191T>C (S45P), respectively (Fig. 4).

Alignment of ND3 proteins from different species in the region of the identified A47T substitution. The arrow indicates the strict conservation of an alanine residue at position 47 in all species assessed.
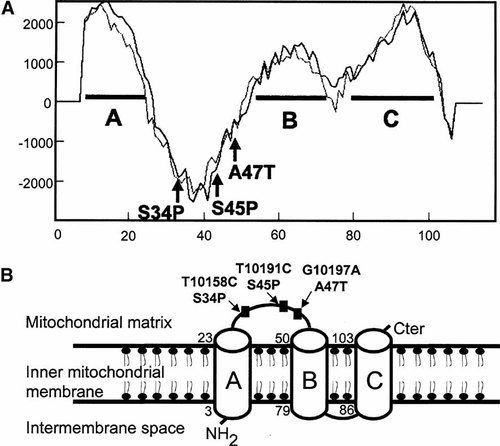
A: Hydropathy analysis of the ND3 subunit. Letters A, B, C design three putative transmembrane helices predicted with TMPred software. B: Predicted transmembrane domains of ND3. Three transmembrane domains A, B, C are predicted according to the TMPRED software. ND3 pathogenic mutations 10158T>C (S34P) and 10191T>C (S45P) including 10197G>A (A47T) in bold are all localized in an extramembrane loop facing the matrix side between A and B transmembrane domains.
Lymphoblastoid cell lines from affected Patients 8 and 12 and the unaffected sister (Patient 9) of family 3 were enucleated and fused with Wal2a ρ° cells to generate cybrid cells. The resulting cybrid cells were subsequently expanded and mtDNA was restored to a normal level in patient cybrid cell lines. Biochemical assays were performed on isolated mitochondria from cybrid cells. Specific enzyme activities in cybrids from Patients 8, 9, and 12 (harboring 97%, 69%, and 100% mutant mtDNA, respectively) showed low complex I activity compared to controls whereas complex III and IV displayed normal values (Table II). Moreover, polarographic analyses of mitochondria isolated from cybrids of cells from Patients 8 and 12 show low malate + pyruvate and glutamate + malate maximal (state III) respiration oxidation rates compared to controls whereas succinate oxidation rates were identical to controls (data not shown).
DISCUSSION
LS is a subacute necrotizing encephalomyopathy frequently associated with complex I deficiency. Several mitochondrial and nuclear mutations in genes encoding subunits of this complex have been already described [Wallace et al., 2006]. Indeed, complex I is composed of approximately 45 polypeptides, 7 of which are encoded by the mtDNA [Hirst et al., 2003]. While mutations have been found in several complex I structural subunit genes encoded by the mtDNA or nDNA, structural gene mutations have not been found in about 60% of complex I-deficient patient [Triepels et al., 2001]. A total of eight mutations of mtDNA complex I genes have been previously reported in young children with LS and isolated complex I deficiency [Kirby et al., 2000, 2003; Taylor et al., 2001, 2002; Chol et al., 2003; Lebon et al., 2003; Ugalde et al., 2003; McFarland et al., 2004; Sudo et al., 2004]. Some of these mutations are recurrent, the more frequent being the 13513G>A mutation in ND5 gene as nine pedigrees carrying this mutation have been reported [Chol et al., 2003; Kirby et al., 2003; Lebon et al., 2003; Sudo et al., 2004]. Two of these mutations are specific to LS (10158T>C, 12706T>C) whereas the others have also been identified in patients with other clinical phenotypes such as MELAS syndrome (13513G>A), progressive mitochondrial disease (10191T>C), or dystonia (14459G>A).
We report here a novel maternally inherited mtDNA mutation, 10197G>A, in three independent families with isolated complex I deficiency and LS or dystonia. This mutation was found to be homoplasmic in the most severely affected patients (children) whereas the mutant load varied from 50% in leukocytes of one healthy mother (Patient 5) to 74% in leukocytes of another affected mother (Patient 8). The clinical phenotype ranged from mild neurological phenotype for two of the three mothers to severe LS for their children. The percentage of mutant mtDNAs must be high before the biochemical and clinical expression threshold is exceeded. For example, complex I activity must be reduced by more than 70% before oxygen consumption or ATP production is perturbed [Letellier et al., 1994; Malgat et al., 1995; Trounce et al., 1996]. Hence, a mutation in an mtDNA complex I subunit would have to reach a relatively high percentage of the cellular mtDNAs before it would significantly impede mitochondrial respiration and energy production, as observed in our families. Most of mtDNA mutations cause a mitochondrial dysfunction when they reach a threshold level greater than 90% mutant DNA in affected tissues. However, the threshold for expression of a complex I deficiency may also depend on the nucleotide position, type of mtDNA mutations, or the presence of additional genetic factors. For instance, patients with the 13513G>A mutation in ND5 gene responsible for LS had unusual low mutant load below 50% responsible for a severe complex I defect and this mutation may act in a dominant manner [Kirby et al., 2003].
Mutations of complex I mitochondrial genes are occasionally found in sporadic cases [Lebon et al., 2003; McFarland et al., 2004] but maternal transmission has also been reported [Kirby et al., 2000; Lebon et al., 2003; Leshinsky-Silver et al., 2005]. In previously reported cases of maternally inherited complex I deficiency, the mothers are generally healthy. Interestingly, in the three families presented here, two of the mothers were mildly affected and presented a relatively high level of mutant mtDNA (50–70% in leukocytes). However, we also observed a 73% mutant load in leukocytes in the 9-year-old unaffected daughter of family 3. The level of mtDNA mutation has been shown to decrease in mitotic tissues with aging as described for the 3243 A>G mutation [Rahman et al., 2001]. Thus, since wild-type mtDNA is selected from dividing blood cells, the mutant becomes progressively more difficult to detect in older patients.
Several lines of evidence support the pathogenicity of the 10197G>A mutation. In our families harboring the 10197G>A mutation, the maternal transmission of the mutation as well as the various mutant loads in affected and unaffected individuals strongly demonstrate the deleterious nature of the 10197G>A mutation. This mutation present on different mtDNA haplogroups may suggest that the 10197 G>A mutation has arisen at least three independent times and it is not a rare lineage-specific polymorphism. We have previously shown that the average conservation index (CI) of 22 well-characterized human pathogenic mtDNA mutations was 93 ± 13% using a series of 39 different species [Ruiz-Pesini et al., 2004]. The CI of the 10197G>A ND3 mutation was calculated based on the same set of 39 species reaching 92.3% (conservation of 36 species except Xenopus laevis, Drosophila melanogaster, and Sciurus vulgaris) in the range of the most deleterious mtDNA mutations and strongly suggests the pathogenicity of the 10197G>A mutation. Furthermore, this defect could be biochemically transferred along with the mutant mtDNAs to ρ° lymphoblastoid cells in cybrid experiments. A scoring approach to assess the pathogenicity of mtDNA variants was applied to the 10197G>A mutation as described [Mitchell et al., 2006] and reached a final score of 37 (maximum score of 40). Hence, the association of the 10197G>A ND3 mutation with an isolated biochemical defect involving complex I and the discovery in three unrelated families with the 10197G>A mutation and a similar phenotype strongly suggest the pathogenicity of this mutation. This mtDNA variant was previously found in a patient associated with an isolated complex I deficiency and Leigh-like syndrome, and cybrid experiments showed a specific alteration of complex I activity [Kirby et al., 2004b]. However, this variant was also identified by Kirby et al. [2004b] in a group of Polynesian individuals in combination with two additional mtDNA variants at positions 12239 C>T and 15746 A>G and was then considered as haplotype polymorphic marker. Both 12239 C>T and 15746 A>G mtDNA variants defining haplogroup B4 were absent in our three families. This patient was also shown to harbor a de novo nuclear translocation between chromosomes 11 and 18 suggesting that the pathogenicity of the 10197G>A mutation was maybe regulated by additional genetic nuclear factors. Several studies have demonstrated that the penetrance of mitochondrial disorders due to mtDNA mutations can be affected by additional nuclear loci. As an example, the homoplasmic 1555A>G mtDNA mutation has been identified in several families with maternally inherited aminoglycoside-induced deafness and variable penetrance due to nuclear-encoded modifier genes [Bykhovskaya et al., 2004; Fischel-Ghodsian et al., 2004].
Mitochondrial genetic defects are thought to be mainly of nuclear origin, especially if symptoms begin during infancy or early childhood and of mtDNA origin if symptoms start during adulthood [Rubio-Gozalbo et al., 2000]. Interestingly, mothers of our three families P1, P5, and P8 (Fig. 1) in our study are mildly affected or unaffected (ages 41, 34, and 37 years old, respectively). Next generation results in early and severe clinical manifestations with early onset and death in several cases (Fig. 1) maybe due to the presence of one or more modifier gene(s) modulating mtDNA mutant expression. Moreover, complex I enzyme activity normal in lymphoblast cells of the unaffected patient P9 of family 3 was significantly decreased in cybrid cells (32% of the control mean) supporting a nuclear involvement that may influence the phenotypic expression of the disease.
Coordinated interactions between the mitochondrion and the nucleus responsible for this large complex with multiple subunits remain poorly understood. The overall structure of complex I is L-shaped with one hydrophobic arm lying in the inner mitochondrial membrane and another peripheral arm extending into the mitochondrial matrix [Guenebaut et al., 1998]. The hydrophobic fragment probably contains the majority of the mtDNA-encoded polypeptides including ND3 [Chomyn et al., 1986; Brink et al., 1987]. Little is known regarding the structural and functional roles of these mtDNA-encoded subunits. Specific deletion of ND2 and ND3 in Neurospora crassa mutants prevents assembly of the membrane arm of complex I [Alves and Videira, 1998]. Furthermore, a patient with severe LS harboring a mutation in ND3 gene at position 10191T>C has been recently shown to be associated with a reduction of complex I assembly [Leshinsky-Silver et al., 2005]. Interestingly enough, the 10197G>A is the third mutation described in the ND3 gene causing LS including the 10158T>C (S34P) and 10191T>C (S45P) mutations. These mutations are located in a relative close proximity in a fragment of less than 15 amino acid residues and may represent a hot spot of mutations (Fig. 4). This fragment is part of the extramembrane loop connecting two putative transmembrane helices of ND3 and strongly suggests that this domain potentially interacts with other structural complex I subunits maybe acting as nuclear modifier genes and require further experiments.
In conclusion we report a novel recurrent ND3 mutation fulfilling canonical criteria for being a pathogenic mutation in three unrelated families. With a cluster of three common mtDNA mutations already described, ND3 gene should be considered as a hot spot of mutations. This report confirms that the systematic analysis of mitochondrial complex I genes yields a high rate of mutations especially in patients with LS.
Acknowledgements
This research was supported in part by the Association Française contre les Myopathies, NIH grants RO1 NS21328, NS41850, AG24373 (D.C. Wallace, V. Procaccio), a grant from the Anna-Geissler Foundation awarded to V.P. We thank D.C. Wallace for his support and Antonio Davila and Lauren Dennis for technical help.




