Abstract
We report on a young woman with Jacobsen syndrome (JBS) who was admitted to our psychiatric department because of a bipolar affective disorder (BPAD). Chromosome analysis was performed due to the fact that she had mental retardation, short stature, and subtle facial anomalies. A deletion of the distal long arm of chromosome 11 was found. A detailed mapping of the deletion breakpoint by quantitative real time PCR revealed a true terminal 11q deletion of approximately 8 Mb corresponding to the karyotype 46,XX,del(11)(q24.2). Polymorphic DNA marker analysis showed that the deletion is located on the paternal chromosome. Additionally, laboratory investigations revealed a low platelet count and magnetic resonance imaging of the brain showed white matter T2 hyperintensities in frontotemporal regions, which are unlikely to result from a demyelinating process as indicated by localized proton magnetic resonance spectroscopy. To our knowledge, this is the first report describing a BPAD in a case with JBS. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Jacobsen syndrome (JBS; 11q deletion syndrome, OMIM no. 147791) is a rare (incidence <1:100,000) but well-known chromosomal deletion syndrome caused by a partial terminal deletion of the distal long arm of chromosome 11. The syndrome was first described by Jacobsen et al. [1973]. The chromosomal deletions are variable in extent but usually involve material distal to band 11q23 [Penny et al., 1995].
Different organ systems can be affected in JBS. The main cause of early infant death is severe cardiac anomaly (50%) [Grossfeld et al., 2004]. Also common are failure to thrive, urogenital anomalies (hydronephrosis, cystic kidneys), digital anomalies (camptodactyly), psychomotor retardation, thrombocytopenia, and pancytopenia. Eye abnormalities include strabismus, atrophy of the optical nerve and coloboma of the iris [Lee et al., 2004]. Some authors also report hearing loss [Fryns et al., 1986; Pivnick et al., 1996].
Several typical craniofacial stigmata are found in Jacobsen patients, such as high palate, prominent forehead, broad nasal bridge, hypertelorism, telecanthus, trigonocephaly, low set dysmorphic ears, a carp shaped mouth, and ptosis. It has been suggested that hemizygous deletions of the homeodomain gene BARX2 may be the cause for these stigmata [Krasner et al., 2000]. The male to female ratio is 1:2.
CLINICAL ANALYSIS
The patient was born as the third child of healthy parents after an uneventful pregnancy. At the age of 9 months mild dysmorphic changes and strabism were noted. At school, she had learning problems and went to a special school from age 8. Today she lives in a sheltered home. Our patient is now 35 years and was first admitted to the psychiatric hospital at the age of 27 years. She presented with a manic psychosis according to the ICD-10 and DSM-V criteria. There was a history of depressive episode of medium severity; accordingly a bipolar affective disorder (BPAD) was diagnosed. She responded well to neuroleptic medication and supportive benzodiazepines. Since that time, seven new manic episodes have occurred.
On physical examination at 31 years, short stature was noted (height: 147 cm; below 3rd centile; weight 68 kg; head circumference 56.5 cm). The blood pressure was 90/60 mm Hg. There were several dysmorphic stigmata: telecanthus (inter canthal distance 4.5 cm), short and broad nasal bridge, a prominent forehead and short fingers (hand length: 16.5 cm) (Fig. 1A,B). On laboratory investigations, a marked thrombocytopenia was noted (104,000/mm3).
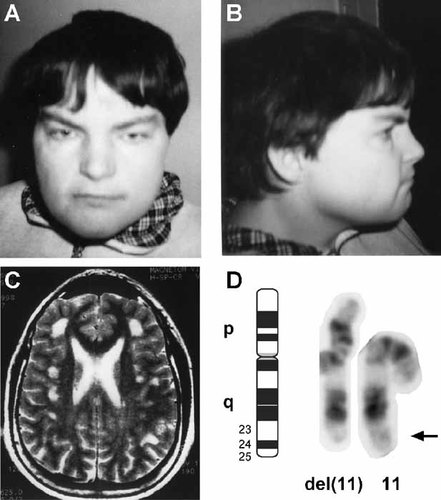
A, B: Patient at the age of 27 years. Note telecanthus and broad nasal bridge. C: Cerebral MRI of the patient. Multiple foci with T2 hyperintensities are present in the frontotemporal grey and white matter. D: Trypsin Giemsa banded chromosome 11 pair of the patient. The del(11)(q24) is clearly visible.
Using the HAWIE-R test, a general IQ of 47 was determined indicating moderate mental retardation. These findings were confirmed by the Benton test.
The parents of the patient reported no other cases of BPAD in the family. A nephew of the patient was described as mentally retarded although an exact diagnosis is not available.
Magnetic resonance imaging (MRI) showed multiple pathological white matter hyperintense signals (T2 and FLAIR sequences) in the frontotemporal region and a mild global brain atrophy (Fig. 1C).
Additional MRI and proton magnetic resonance spectroscopy (MRS) were performed at 2.0 T (Magnetom Vision, Siemens, Erlangen, Germany). Fully relaxed short-echo time proton MR spectra were acquired using a single-voxel STEAM localization sequence as described previously [Frahm et al., 1990]. User-independent spectral evaluation was based on the fully automated program LCModel [Provencher, 1993]. Major detectable metabolites are the neuroaxonal markers N-acetylaspartate and Nacetylaspartylglutamate (tNAA), creatine and phosphocreatine (tCr), choline-containing compounds (Cho), the glial marker myo-inositol (Ins) and lactate (Lac). Previous investigations of regional age dependencies of cerebral metabolites in human brain [Pouwels et al., 1999] provided age-matched controls for the evaluation of abnormal metabolite concentrations.
In the gray matter we found a slight reduction of all metabolites: tNAA to 6.6 mM (normal 9.1 ± 0.6 mM), tCr 4.6 mM (normal 6.5 ± 0.4 mM), Cho 0.9 mM (normal 1.2 ± 0.3 mM), and Ins 3.0 mM (normal 4.4 ± 0.6 mM). These findings are consistent with a corresponding loss of cellular density and may reflect the patient's mild brain atrophy and moderate mental retardation.
In the affected white matter there was a reduction of Ins (2.7 mM; normal 4.4 ± 0.5 mM) and tNAA (6.4 mM; normal 8.2 ± 0.6 mM), while tCr and Cho were normal. Lac was not detectable in either white or gray matter. These grey matter abnormalities do not support the presence of a demyelinating process, which has previously been characterized by elevated Cho and reduced tNAA [Wilken et al., 2003]. Instead, the decreased concentration of Ins suggests a partial loss of astrocytes.
CYTOGENETIC AND MOLECULAR ANALYSIS
Due to the fact that our patient had dysmorphic signs, a chromosomal analysis on peripheral blood lymphocytes was carried out. Unexpectedly, the karyotype analysis revealed a deletion of the distal long arm of chromosome 11 with break in band q23.3∼24 (46,XX,del(11)(q23.3∼24?) (Fig. 1D)). The parents' karyotype was normal, indicating a de novo deletion in the patient.
The breakpoint on chromosome 11q was mapped by a quantitative real-time polymerase chain reaction (PCR) approach as described previously [Boehm et al., 2004]. In order to fine map the deletion breakpoint in our patient, we designed primers for a set of physically mapped amplicons (100–200 bp) covering the long arm of the human chromosomal region 11q in approx 1 Mb intervals (see the online Table I at http://www.interscience.wiley.com/jpages/1552-4825/suppmat/index.html). In the proband's DNA, a hemizygous loss of all markers of the chromosomal region distal to 126.02 Mb was found. The deletion breakpoint could be further refined to a 844 kbp region between 126.020 and 126.864 Mb, which corresponds to the cytogenetic band 11q24.2. Taking 134.45 Mb as the total length of chromosome 11, approximately 8 Mb are hemizygously deleted in the patient. Subtelomeric imbalances of the other 22 chromosomes were excluded by quantitative PCR genotyping of subtelomere amplicons (data not shown in Table I) [Boehm et al., 2004]. The parental origin of the deletion was determined using highly polymorphic STS markers (D11S1353, D11S874, D11S912) from within the deleted region. The D11S874 and D11S912 alleles were informative. The patient's DNA showed only maternal alleles in the deleted region, indicating that the deletion is on the paternal chromosome. D11S4090 was used as a control from the non-deleted portion of chromosome 11.
DISCUSSION
We describe a patient with the typical clinical presentation of JBS (including mental retardation, short stature, hypertelorism, and low platelet count) and a bipolar affective disorder (BPAD). An association that, to our knowledge, has not been described before.
Our patient is of short stature, which is a common finding in JBS. Low IGF-1 levels and cryptorchism in affected males indicate a defect in the growth hormone axis in JBS [Haghi et al., 2004]. According to Grossfeld et al. [2004] the minimal deleted region for short stature in JBS ranges from 124.2 Mb (from the p telomere) to the q telomere. Patients with a 6.8 Mb deletion (distal to D11S1351 corresponding to a breakpoint at 128.056 Mb, NCBI database) showed platelet disorder, pylorus stenosis and mental retardation, but no short stature. The breakpoint in our patient with short stature is distal to 126.02 Mb. Under the assumption that short stature is 100% penetrant, this would suggest that the genomic region between 126.02 and 128.056 Mb may be responsible for short stature in JBS (see the online Fig. 1 at http://www.interscience.wiley.com/jpages/1552-4825/suppmat/index.html). The deletion in our patient includes the FLI1 gene, which encodes an ets domain transcription factor essential for hematopoesis and which has been suggested to be involved in thrombopenia [Raslova et al., 2004].
Penny et al. [1995] stated that larger deletions with breakpoints centromeric to STS marker D11S924 (approximately 119 Mb from the p telomere) are preferentially on the maternal chromosome while smaller deletions with more distal breakpoints are usually paternal. Our case with a rather small deletion of paternal origin agrees with this observation. It is believed that a parent of origin effect may play a role in the etiology of 11q deletion syndrome.
The white matter changes seen on MRI in our patient have also been observed in some other patients with JBS [Wardinsky et al., 1990; Ono et al., 1996], although systematic studies have not been conducted. However, by MRS we could demonstrate that these changes are not due to a demyelinating process but rather suggest a partial loss of astrocytes. It thus remains unclear whether these findings represent manifestations of the JBS phenotype.
To our knowledge, this is the first case of JBS, which is associated with BPAD. Recently, a susceptibility locus for psychoses was mapped to distal 11q [Lewis et al., 2003]. One candidate gene for BPAD in this distal 11q region, which is also deleted in our patient, is the Beta-1,3-glycoronyltransferase gene (B3GAT1) [Jeffries et al., 2003]. B3GAT1 is expressed almost exclusively in the brain, where its expression is temporally and spatially regulated. It is the main glucuronyltransferase involved in the biosynthesis of the carbohydrate HNK-1 epitope, which is present on a number of adhesion molecules [Jeffries et al., 2003]. B3GAT1 is located in the subtelomeric region in band 11q25 (133.77 Mb) less than 1 Mb from the telomere. Interestingly, mice with a knock-out of the murine B3GAT1 gene showed impaired synaptic plasticity and spatial learning as well as reduced long term potentiation at certain synapses in the hippocampus [Yamamoto et al., 2002]. Lewis et al. [2003] reported linkage of schizophrenia to several 11q distal markers, and Jeffries et al. [2003] reported a pedigree in which a (6;11) translocation cosegregated with psychotic illness. The 11q breakpoint in this family was situated in the immediate vicinity to the telomere. These data suggest that genes in the very distal part of chromosome 11 may be involved in susceptibility to schizophrenia and related psychoses and point strongly to B3GAT1 as a candidate genes for these disorders. A more careful analysis of the neurological phenotype of the B3GAT1 knock-out mice seems to be warranted.
B3GAT1 is assumed to be deleted in all patients with Jacobsen syndrome and true terminal 11q deletions. If hemizygosity for B3GAT1 plays a role in bipolar disorders and/or schizophrenia, all JBS patients should have an elevated risk to develop psychotic illness as they reach adulthood. However, the majority of JBS patients reported to date are children, approximately 25% of patients die before the age of 2 and only a few adult patients are known. In our patient the deletion is rather small only encompassing the terminal 8 Mb of 11q. Patients with smaller deletions generally present with a milder phenotype. In severely affected patients, that is, patients with larger deletions, the signs of psychotic changes may be misinterpreted and/or be masked by the other symptoms of JBS. Interestingly, Neavel and Soukup [Neavel and Soukup, 1994] reported a proband with a small 11q deletion (del(11)(q24.2)) and schizophrenia adding further evidence that there might be an association between terminal 11q deletions and schizo-affective disorders.
Since subtle subtelomeric deletions might be more common than generally assumed, it might be interesting to search for subtelomeric deletions of 11q in a larger group of patients with BAPD. Subtelomeric deletions in patients with psychiatric illnesses might not be uncommon. Recently, a subtelomeric deletion in the long arm of chromosome 4 was reported in a patient with mental retardation and schizo-affective disorder [Pickard et al., 2004].
JBS is so far the only chromosomal deletion syndrome for which a genetic predispostion has been suggested. Jones et al. [1994, 1995] reported the colocalization of JBS breakpoints with the inherited folate-sensitive site FRA11B. It is thus tempting to speculate that there is an expanded CCG repeat in the family of our patient and that the nephew also has JBS. Even though most cases of JBS do not seem to be associated with a break at FRA11B [Michaelis et al., 1998; Tunnacliffe et al., 1999], a study by Jones et al. suggested that these cases might have breaks in more distals fragile sites containing CCG-repeats [Jones et al., 2000]. However, fragile site analysis in parents of patients with smaller 11q deletions failed to detect such sites [Grossfeld et al., 2004].
However, with BAPD and schizo-affective disorders having a high incidence in the general population, one has to bear in mind that the association seen in our patient could be purely coincidental.
Acknowledgements
We thank Sabine Herold and Ursula Dybowsky for excellent technical assistance.




