A new syndrome of congenital generalized osteosclerosis and bilateral polymicrogyria
Abstract
We report a 29-week male fetus with healthy consanguineous parents. He showed a severe sclerosing bone disorder affecting all skeletal elements, resulting in insufficient modeling, generalized densification, and fragility of the skeleton. This skeletal dysplasia was associated with an abnormal craniofacial development (hypertelorism, severe microretrognathia, cleft palate, absent epiglottis, reduced number, and mineralization of teeth buds) and abnormal terminal phalanges. Neuropathologic examination showed bilateral fronto-parietal cerebral polymicrogyria. This syndrome appears to represent a new variant of congenital sclerotic bone disorder of unknown origin. Autosomal recessive inheritance is possible. © 2005 Wiley-Liss, Inc.
INTRODUCTION
Osteosclerotic bone disorders are a heterogeneous family of diseases characterized by an imbalance between osteogenesis and osteoclasis, leading to dense and often overgrown skeleton. Radiographically, osteosclerosis is suggested by the presence of an increased width of trabecular bone, contrasting with osteopetrosis, where a generalized increase in bone density with bony encroachment into the medullary cavities is observed. Chronology (congenital, infantile, and adult), topography of the modeling defect (generalized, predominating on skull basis, facial bones, and/or other parts of the body), biochemical anomalies, and associated anomalies allow the partition of sclerosing disorders into several genetic and clinical types [Spranger and Maroteaux, 1990].
Brain polymicrogyria is a malformation of cortical development characterized by an excessive number of small gyri with abnormal cortical lamination. It can appear as either a focal lesion or a more widespread cortical abnormality [Barkovich et al., 1995]. The presence of bilateral symmetric polymicrogyria is often taken to suggest a genetic etiology, and such multiple syndromes have been described. These syndromes have distinct clinical and radiological presentations and include sporadic types (bilateral frontal polymicrogyria [Guerrini et al., 2000] and bilateral para-sagittal parieto-occipital polymicrogyria [Guerrini et al., 1997]), and some familial forms, such as bilateral perisylvian polymicrogyria [Guerreiro et al., 2000; Villard et al., 2002] and bilateral fronto-parietal polymicrogyria (BFPP) [Chang et al., 2003]. In this latter, autosomal recessive form, mapped to chromosome 16q12.2-21 [Piao et al., 2002] polymicrogyria is typically present with a descending anterior–posterior gradient of severity, the fronto-parietal regions being the most significantly affected.
We report on a case of sclerosing bone disorder of very early onset associated with a bilateral fronto-parietal polymicrogyria that may represent a new radiological and clinical form within this family of disorders.
PATIENT REPORT
The patient is the second child of healthy consanguineous parents (first cousins) from Mauritania. Family history was not contributory. At 23 weeks of gestation, a routine ultrasonographic examination revealed hypertelorism, cleft palate, severe microretrognathia, abnormally shaped ribs, and a narrowed thorax. At 29 weeks of gestation, cranial MRI revealed a small brain with abnormally short corpus callosum and poorly defined sulci, suggesting an abnormal gyration pattern (Fig. 1a,b). Infectious causes were ruled out. Fetal karyotype was 46, XY. Parents elicited termination of pregnancy.

MRI images of the patient's brain showing poorly defined sulci in the frontoparietal region, bilaterally (arrows) (a), and the corpus callosum lacking genu et splenium (arrowheads) (b). Comparison with brain MRI from a healthy 29 weeks-old fetus (c, d).
At necropsy, the boy weighted 1.340 g (75th centile), his length was 37 cm (10th–25th centile), and his head circumference was 28.5cm (90th centile). The left humerus and both femora were broken during delivery. He showed facial dysmorphism: frontal bossing, hypertelorism, downslanted palpebral fissures, upturned nose, long flat philtrum with a median groove, microstomia, and severe microretrognathia (Fig. 2a,b). Exophtalmia was not observed. Auditory meati were narrowed. The palate was cleft posteriorly and the epiglottis was absent. Gums were broad and thickened. Two para-median whitish dental cysts were present on the upper gum, and the lower gum had a median notch. Hands were slender, with long fingers ending with short and broadened, spatulate terminal phalanges (Fig. 2c). Thumbs and halluces were large.
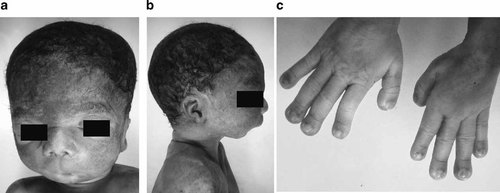
External phenotype of the patient: note the frontal bossing, hypertelorism, downslanted palpebral fissures, upturned nose, long flat philtrum, microstomia (a), severe microretrognathia (b). Hands are slender, with long fingers ending with short and broadened, spatulated terminal phalanges (c).
Radiological examination showed increased biparietal diameter (>90th centile) and extreme sclerosis of the skull basis and facial bones. Mandibular dental germs were not visible. Long bones (including extremities) were irregularly shaped, with insufficient metaphyseal wedging (Fig. 3a). Diaphyseal bones were extremely dense, with thick cortex and an absent medullar canal. The upper thorax was narrow. The ribs and the clavicles were thin but regularly shaped (Fig. 3b). The vertebral bodies were sclerotic and slightly flattened (Fig. 3c). The first metacarpals and the distal phalanges were short, the latter showing a terminal tufting (“mushroom profile”) (Fig. 3d). Bone age was delayed, corresponding to about 23 weeks of gestation [Stempfle et al., 1995].
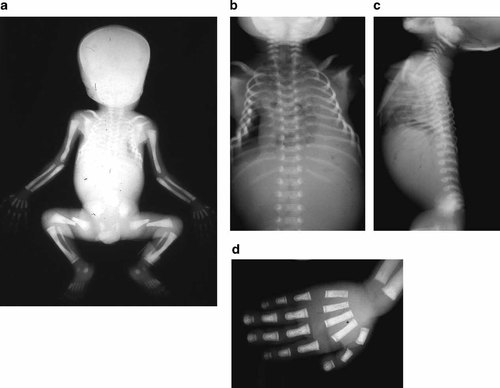
X-ray examination showing: (a) extreme sclerosis of the skull basis and of the facial bones. b: Long bones and spine are irregularly shaped with insufficient metaphyseal wedging. Diaphyseal bones are extremely dense, with absent medullar canal. c: The chondro-costal junction is angulated. d: The first metacarpals and the distal phalanges are short, the latter showing a terminal tufting (mushroom profile).
Macroscopic examination of the brain showed an abnormal gyral pattern and Y-shaped rolandic sulci. The corpus callosum lacked genu and splenium. Microscopically, a bilateral symmetric frontoparietal and, to a lesser extent, temporal polymicrogyria was evident (Fig. 4). The ventricular system was normal, as was the cerebellum.
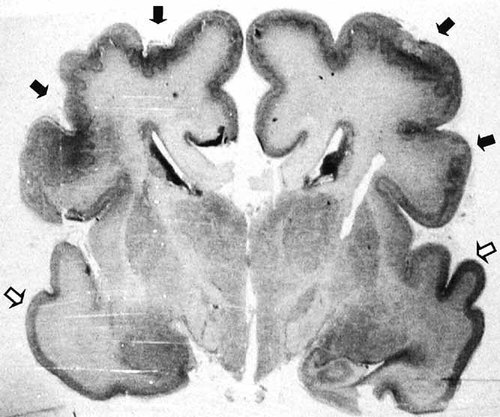
Microscopic examination of a coronal section of the brain, showing bilateral parietal polymicrogyria (black arrows). The temporal cortex is normal (white arrows).
Microscopic examination of the femur showed a normal architecture and cellularity of the epiphyseal cartilage and growth plate. Cortical bone was thin and irregularly lined. Trabecular bone was characterized by an irregular and insufficiently mineralized matrix containing numerous osteocytes, lined by numerous osteoblasts and osteoclasts. It was more compact than normal: the medullar cavity was not developed, and hematopoiesis was poor. Membranous ossification was studied on a fragment: the diploe. It appeared thickened and hypercellular, as was also the femoral trabecular bone (Fig. 5). Microscopic examination of the mandibula showed similar alterations of bone structure, and widened symphysis with a large median cartilaginous nucleus. There were only two dental germs, with several cuspids (primary molars?) corresponding to the gingival nodules. In the maxillary bone, six germs were found: two incisors, one canine, one medially displaced tooth (canine or incisor), and one molar. The micro-structure of all dental germs was normal, but mineralization was severely delayed, and they appeared abnormally oriented.
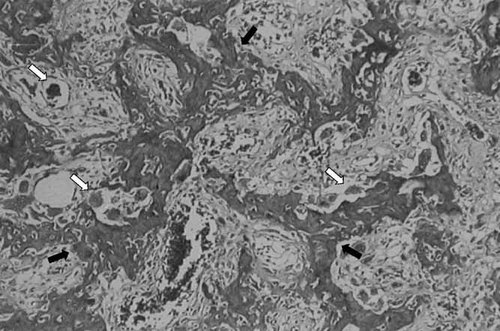
Microscopic examination of diaphyseal trabecular bone showing reticular aspect of trabeculae (black arrow), hypercellularity and irregularity of the bone matrix. An increased number of osteoclasts (white arrow) and osteoblasts are observed around the trabeculae. Hematopoiesis is reduced.
Macroscopic and microscopic examinations of other internal organs were unremarkable, as were the placenta and cord. X-ray examination of the hands and skulls of both parents were normal.
DISCUSSION
The boy reported here, born to consanguineous parents, showed a severe sclerosing bone disorder affecting all skeletal elements and resulting in insufficient modeling, in generalized densification and fragility of the skeleton, and in very abnormal terminal phalanges. This skeletal dysplasia was associated with abnormal facial and dental development (hypertelorism, severe microretrognathia, cleft palate, absent epiglottis, and abnormal teeth buds). His brain showed bilateral fronto-parietal polymicrogyria and a hypoplastic corpus callosum, lacking genu and splenium.
Potential environmental etiologies of polymicrogyria have been reported [Barkovich et al., 1995]. A possible explanation for our patient's cortical anomalies could have been an antepartal anoxicischemic disruption, but the mild extent of laminar necrosis in the territory of the median cerebral arteries does not support this hypothesis. Furthermore, bilateral symmetric polymicrogyria present without signs of laminar necrosis in other cortical areas, and the association with a dysgenetic corpus callosum led us to hypothesize a genetic etiology for this cortical dysplasia.
Several reports may be discussed in the differential diagnosis of congenital generalized bone sclerosis and cerebral abnormalities. Lethal osteosclerotic bone dysplasia, or Raine syndrome (MIM 259775), was described by Raine et al. [1989] in a female fetus with microcephaly, exophthalmos, hypoplastic midface, gum hyperplasia, cleft palate, and osteosclerosis. To date, 14 cases of Raine syndrome have been reported, allowing a rather precise delineation of the phenotype [Al Gazali et al., 2003; Hulskamp et al., 2003]. The characteristic face of Raine syndrome consists of a narrow prominent forehead, hypertelorism, proptosis, distinct midfacial hypoplasia with downward eye slant, very short and flat nose, carp-shaped mouth, gum hyperplasia, and severe microretrognathia. The radiological findings in Raine syndrome include generalized osteosclerosis of all bones and the base of the skull with cortical hyperostosis, metaphyseal flaring, and irregular appositional bone formation along the diaphyses of the long tubular bones. These characteristic features were absent in our patient, in whom the diaphyses of the long bone and the metaphyses are regularly lined and well demarked from the surrounding soft tissues. In Raine syndrome, the ribs and clavicles are short and the vertebral bodies slightly flattened. The hands show brachytelephalangy with short terminal phalanges, quite different from the remarkably broad phalanges of our patient. Histologically, the tubular bones show normal endochondral ossification, narrow medulla, compaction and thickening of the diaphyseal cortex, and irregular trabecular bone formation with osteoblastic and osteoclastic activity around amorphous deposits and calcifications. In our case, on the contrary, the characteristic lesion was the hyperplasia of an abnormal endochondral bone, and hypoplasia of the cortical periosteal bone, without extra-osseous osteoid deposits [Kan and Kozlowski, 1992]. Desmosterolosis (MIM 602398), a metabolic abnormality resembling Raine's phenotype has been searched for and excluded. [FitzPatrick et al., 1998]. Al Mane et al. [1996] first demonstrated that intracranial calcifications of unknown etiology are a component of Raine syndrome. Widespread focal calcifications were evidenced in the periventricular white matter and basal ganglia with some meningeal calcifications as well, and these were thought to correspond to the histologic calcifications observed by Kan and Kozlowski [1992]. These features suggested the possibility of a generalized disturbance of calcium metabolism. In no instance have the calcifications been associated with polymicrogyria.
Dysosteosclerosis (MIM 224300) is a rare autosomal recessive bone dysplasia associated with neurodevelopmental deterioration and optic atrophy due to cranial nerve compression. X-linked inheritance has been described [Pascual-Castroviejo et al., 1977]. In this syndrome, which expression starts in infancy, sclerosis is associated with progressive metaphyseal expansion and alteration of bone density. The early craniotubular bone modeling and clinical presentation resemble osteopetrosis. Affected individuals have dysmorphic features with a round face, sagging cheeks, and a prominent forehead. Dentition is abnormal. There is sclerosis of the skull base, the ribs (that are wide), clavicles, scapulae, and mid-diaphyses. The metaphyses show progressive expansion and, as in the spine, develop sclerotic islands in areas of relative radiolucency. These irregularities of bone density were not observed in our patient. There is mild platyspondyly with wide intervertebral spaces. The vertebral bodies are small with irregular end plates and pronounced anterior notches. The tubular bones are short with progressive bowing, and fractures are a complication [Elcioglu et al., 2002]. Chitayat et al. [1992] reported a girl with a clinical and radiological diagnosis of dysosteosclerosis who presented diffuse intracerebral calcifications. Although our case shows some radiographic similarities with other severe osteosclerotic dysplasias, these usually present with limb shortness and without neurological involvement, with the exception of the above mentioned Raine syndrome [see the table by Brodie et al., 1999].
Some other patients with osteosclerosis and abnormal brain structure have been recorded. Lehman et al. [1977] described a mother and daughter with generalized osteosclerosis, multiple lumbar and thoracic meningoceles, an empty sella, hypoplasia of the cerebellar vermis, and small cerebral gyri, the socalled lateral meningocele syndrome [Gripp et al., 1997]. In the report of El Khazen et al. [1986], a lethal condensing bone disorder was detected in utero in two sibs. The bones were brittle and fractures occurred. Neuropathological examination revealed hydrocephalus and an intense gliosis throughout the cortex and white matter, with an extensive loss of neurons. Numerous axonal swellings were observed in the cortex, white matter, and brainstem. Calcifications were occasionally seen. The cerebellum was hypoplastic. Al-Gazali et al. [1998] reported a consanguineous Pakistani family where 10 infants suffered from the same condition. The only well-described child showed macrocephaly, downslanted palpebral fissures, depressed nasal bridge, and micrognathia. Brain imaging revealed a huge interhemispheric cyst communicating with the lateral ventricles and causing hydrocephalus, callosal agenesis and cerebellar hypoplasia. Skeletal survey showed sclerosis of all bones with wide metaphyses.
Our patient's phenotype is not consistent with any of these syndromes because of the peculiar brain abnormalities found in the lateral meningocele syndrome (thoracic meningoceles), and in the patients reported by El Khazen et al. [1986] and by Al-Gazali et al. [1998] (hydrocephalus and cerebellar hypoplasia).
We were unable to find any murine model showing bone sclerosis and abnormal brain structure (Mouse Genome Informatics, The Jackson Laboratory, USA, http://www.informatics.jax.org/). Interestingly, osteopetrotic (op/op) mice defective in producing functional macrophage-colony-stimulating factor (M-CSF) show abnormal brain development. The numerical density of microglial cells was found to be reduced by 47% in the corpus callosum, by 37% in the parietal cortex, and by 34% in the frontal cortex of mice mutant at the op locus, which are totally devoid of M-CSF [Wegiel et al., 1998; Sasaki et al., 2000].
These data, with the above-mentioned reports of patients presenting structural brain abnormalities and sclerosing bone disease, suggest that alterations in still unidentified (metabolic?) pathways might cause brain-and-bone anomalies, as in peroxisomal disorders, and that this association should be searched for. The combined skeletal and cerebral pattern of anomalies observed in our patient appears to be clearly different from the other osteosclerotic syndromes with CNS involvement. Because of parental consanguinity, an autosomal recessive mode of inheritance is possible. Further clinical, radiological, and histological data are needed to clarify whether the different reported patients correspond to diversely severe phenotype of a unique syndrome, or show causally distinct entities.




