Prenatal death in Smith–Lemli–Opitz/RSH syndrome
To the Editor:
Smith–Lemli–Opitz (RSH) syndrome is an autosomal recessive disorder involving virtually all organ systems. Severity varies widely, but all degrees of severity are due to the disturbance of the same genetic/biochemical system involving a deficiency of cholesterol [Irons et al., 1993]. The enzyme 7-dehydrocholesterol reductase, which is abnormal in RSH syndrome, catalyzes the last step of endogenous cholesterol synthesis [Moebius et al., 1998; Wassif et al., 1998; Waterham et al., 1998]. The gene, DHCR7, which encodes this enzyme, is located on chromosome region 11q13. Some 100 different mutations in the DHCR7 gene have been identified in more than 200 patients with the syndrome [Opitz, 2001]. Cholesterol is a precursor of steroid hormones and bile acids. It is an important component of myelin, mitochondrial and cell membranes [Opitz, 2001; Opitz et al., 2002]. Decreased 7DHCR activity leads to cholesterol deficiency and subsequent accumulation of the precursor molecules 7- and 8-dehydrocholesterol. Prenatally, there is a direct correlation between the severity of the phenotype and 7DHC levels in the amniotic fluid. The clinical condition can range from prenatal death with malformations of multiple organ systems to minimal physical effects and only mild behavior problems and mental impairment in children and adults [Nowaczyk et al., 1999, 2001]. Malformations observed in the RSH syndrome include Y-shaped 2–3 toe syndactyly, polydactyly, unilobate lungs, renal dysplasia, or agenesis, Hirschsprung disease, complex cardiac malformations, cataracts, central nervous malformations such as microcephaly and agenesis of corpus callosum, oropharyngeal malformations (cleft palate), genital ambiguity in genetic males, and facial abnormalities such as anteverted nostrils, micrognathia, and apparently low-set ears [Smith et al., 1964; Cunniff et al., 1997; Kelley, 2000].
Opitz et al. [2002] described three cases studied pathologically. Case 1 involved a spontaneous miscarriage of an approximately 19-week gestational age female fetus. Ultrasonography had demonstrated an enlarged third ventricle, thickening of the myocardium and oligohydramnios. Autopsy documented a large secundum atrial septal defect, muscular ventricular septal defect, hypertrophy of the left ventricle and enlargement of the right ventricle. The severely hypoplastic lungs were unilobate with an incipient fissure on the right. Hepatomegaly, bilateral renal agenesis with rudimentary ureters, and a bifid uterus were identified. The umbilical cord had three vessels.
Case 2 was confirmed by gas chromatography mass-spectrometry of amniotic fluid. The cholesterol content was 6.2 mg/ml; 7- and 8-dehydrocholesterol levels were 2.2 and 0.9 µg/ml, respectively. The stillborn male fetus was delivered at 22 weeks of gestation. Ultrasonography at 21 weeks had shown short limbs, micropenis, and possible hydrocephalus. Autopsy findings included fused eyelids, a nose with a single midline nasal pit and choanal atresia, high arched palate with broad alveolar ridges, postminimus of the left hand, postaxial polydactyly of the feet, syndactyly of the 2nd and 3rd, and 5th and 6th toes bilaterally, and nuchal edema. The external genitalia were female. The left lung was unilobate and the right lung was bilobate. The brain was a small solid mass (OFC 17 cm, more compatible with atelen/aprosencephaly than cebocephaly). The umbilical cord had two vessels.
Case 3 was a term, live born infant. A short umbilical cord, cleft palate and abnormal oropharynx were noted at delivery. The infant had upward slanting palpebral fissures, small pupils, cataracts, a flattened nose, and a small tongue, mouth and mandible. The ears were angulated posteriorly. The infant had bilateral 2/3 toe syndactyly and polydactyly of both second toes, 3rd degree hypospadias, and unilateral cryptorchidism. The diagnosis was confirmed on the basis of hypocholesterolemia (19 mg/dl) and hyperdehydrocholesterolemia (180 µg/ml). At 61 days of life, cholesterol treatment had increased the cholesterol levels to 55 mg/dl, but the infant had no psychomotor development. He developed hypertension, reflux, delayed gastric emptying, and suffered numerous upper and lower respiratory infections which ultimately lead to his death at age 7.5 months.
Angle et al. [1998] reported an atypical case of RSH syndrome with hydrops, uncharacteristic facial appearance, and absence of 2/3 toe syndactyly in a 46,XY fetus who died at birth. Antenatal ultrasonography was abnormal and maternal uE3 levels were low. Cultured skin fibroblasts showed elevated 7DHC levels and abnormalities of cholesterol synthesis.
In the atypical, but biochemically confirmed case of Seller et al. [1995] there was oligohydramnios with severe Potter-sequence changes, webbing of axillae and crura (fetal akinesia), small right thumb and severe hypoplasia of right 5th finger, absence of left 5th finger, partial cutaneous syndactyly of toes 2/3 bilaterally, female external genitalia with testes and ducts, agenesis of right kidney and ureter and severe hypoplasia of the left, unilobate left lung, bilobed right lung, one pair of cervical ribs, and non-ossification or absence of vertebrae S4 and S5. Thus, postaxial oligodactyly may be the developmental equivalent of postaxial polydactyly.
In the biochemically confirmed infant of Ness et al. [1997] there was, in addition to typical malformations, biventricular myocardial hypertrophy, PDA, aortic coarctation, bicuspid aortic valve, hepatomegaly with severe cholestasis, early septal fibrosis, marked bile staining of meninges with strikingly abnormal gyral pattern, mild hydrocephalus with porencephaly, absence of corpus callosum, and a hypoplastic cerebellum. Histologically there was an abnormality of cortical migration with four instead of six layers, and a severe lack of myelination. Also noted were depletion of thymocytes, enlarged hyperchromatic nuclei of the pancreatic islet cells (more about which below). LDL receptor levels in brain were upregulated as compared to control samples. This infant survived, profoundly impaired and jaundiced, until 2 months.
Recently, Nowaczyk et al. [2003] reported on a 17½ (?) week male fetus with oligohydramnios and Potter sequence, multiple minor anomalies, cleft of posterior palate, post-axial polydactyly of feet, partial cutaneous syndactyly of toes 5 and 6, ambiguous external genitalia, absence of right kidney and hypoplasia of the left, polysplenia, bilobed right and unilobate left lung, AV septal defect, absent corpus callosum, and Dandy Walker anomaly. Testes were present in this 46,XY fetus whose mother carried the IVS8-1G → C and father the W151X mutation.
We report our experience with Baby P, a pre-term male infant at 34 weeks of gestational age by ultrasonography. The 27-year-old gravida 2, para 1 mother received prenatal care. Fetal ultrasound studies were performed at 19, 27, 30, and 33 weeks of gestation. Known prenatal fetal anomalies included severe hydrops and intrauterine growth restriction, fetal akinesia and an atrioventricular canal and possible coarctation of the aorta. Karytotpye was 46,XY. The infant was delivered by repeat Cesarean section, with Apgar scores of 1 at 2 and 5 min. The infant had joint contractures, ambiguous genitalia, postaxial polydactyly of hands, second and third toe syndactyly, and pronounced hydrops, and died at age 23 min (Figs. 1-5). The autopsy findings are summarized in Table I and illustrated in Figures 6 and 7.
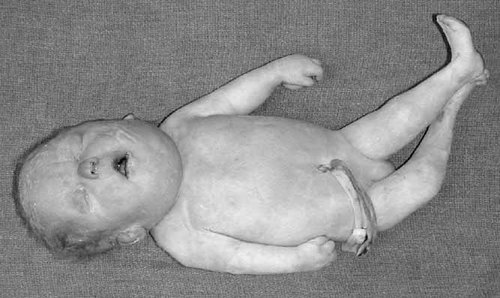
Baby P. Notice narrow forehead, low-set ears, micrognathia, joint contractures and dorsal dislocation of right hip.
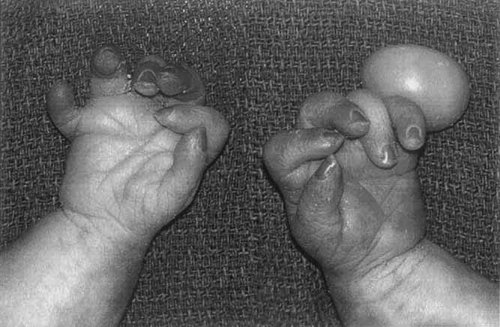
Postaxial polydactyly of hands with torsion of the pedicle of the left 6th digit, camptodactyly, and bilateral single palmar creases.
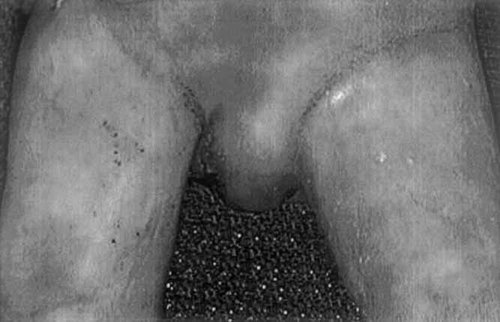
Ambiguous genitalia with the left testicle descended into the labial-scrotal fold and the right testicle in the inguinal canal. The karotype was 46,XY.
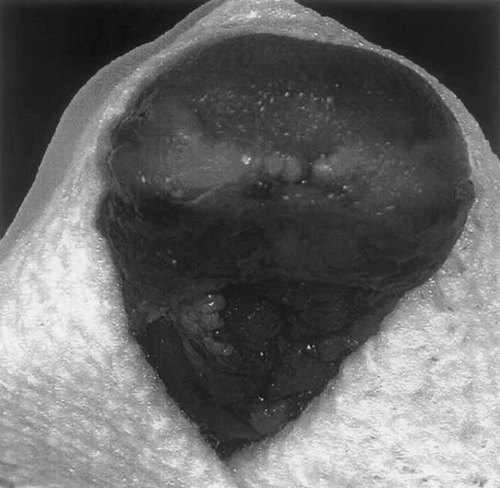
Lack of formation of ventral tongue.
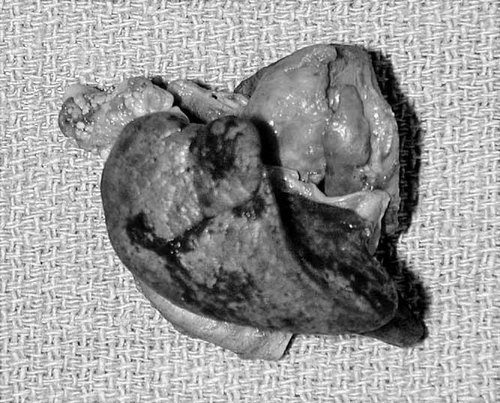
Pulmonary hypoplasia. The right lung, 5.0 g, had one lobe with an incomplete fissure. The left lung 4.0 g (not pictured) was unilobate. The inferior half the heart has been cut away.
| Musculoskeletal system | Postaxial polydactyly of hands |
| Left sixth digit with torsion of pedicle | |
| Flexed fingers, bilateral (camptodactyly) | |
| Simian crease, bilateral | |
| Fixed elbow contractures, bilateral | |
| Dorsal dislocation of the right hip | |
| Hyperextended, fixed right knee | |
| Vertical talus and metatarsus adductus | |
| Two-three toe syndactyly, bilateral | |
| Dorsal placement of the 4th right toe | |
| Axial webbing | |
| Face | Narrow forehead |
| Low-set ears, rotated more than 45° posteriorly | |
| Micrognathia | |
| Mouth | Lack of formation of the ventral tongue |
| Adherent frenulum | |
| Cardiovascular system | Atrioventricular canal |
| Single atrioventricular valve with 3 cusps overriding the left ventricle | |
| Severe preductal coarctation of the aorta | |
| Large atrioventricular septal defect | |
| Overriding pulmonary artery | |
| Right ventricular hypertrophy and enlargement | |
| Genitalia | Ambiguous genitalia |
| Left testicle descended into the labial-scrotal fold | |
| Right testicle in the inguinal canal | |
| Failure of fusion of scrotal raphé | |
| Urinary tract | Hypoplastic right kidney, weight less than 0.1 g |
| Left kidney, weight 2.5 g | |
| Unremarkable ureters | |
| Three-vessel umbilical cord | |
| Pulmonary | Pulmonary hypoplasia |
| Unilobate left lung, 4.0 g | |
| Right lung 5.0 g, one lobe with an incomplete fissure | |
| Potter sequence | Hypoplastic kidneys and lungs as described above |
| Serous pleural effusions, 26.6 cc right, 25.5 cc left | |
| Moderate anoxic brain injury | |
| Potter facial sequence: low set ears, broad compressed upturned nose, | |
| receding chin, fetal akinesia | |
| Symmetric intrauterine growth restriction | Crown-heel length 35.4 cm (expected: 41.1 cm) |
| Foot length 5.6 cm (expected: 6.5 cm) | |
| Brain to liver ratio 3.7:1 (expected 3.0–5.0:1) |
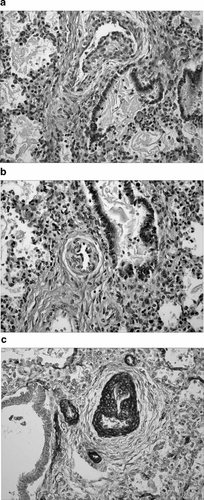
Histologic sections of lung. a: A pulmonary artery with an irregular proliferation of smooth muscle, producing a “mound” that extends into the lumen. b: A second pulmonary artery with the same histologic findings. c: Smooth muscle actin stain demonstrating the proliferation of smooth muscle.
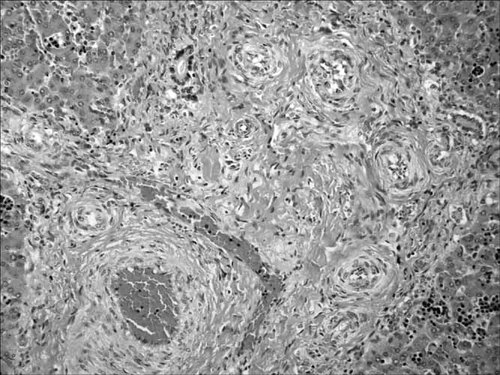
Histologic section of the liver showing vascular changes similar to those identified in the lung.
Sections of the left kidney showed normal architecture. The right kidney was composed of a single pyramidal ray with 2–3 glomeruli. Thickened arterioles extended to the cortical surface. The ureters were identified, and unremarkable. Sections of the lungs showed alveolar spaces in the saccular stage of development. Aspirated squamous cells were identified. There was an irregular proliferation of smooth muscle, producing “mounds” that extended into the lumen of the pulmonary arteries. The lumina were lined completely by endothelium. Reactive endothelial cells and an increased number of fibroblasts were identified (Fig 6a,b). Elastin and smooth muscle stains confirmed the proliferation of smooth muscle, and a focal loss of elastin in these vessels (Fig. 6c). This is a documented pattern of vessel development in fetal lung but one greatly accentuated in this case [Wigglesworth and Singer, 1998]. Similar vascular changes were seen in the liver (Fig. 7). Sections of the brain showed moderate acute anoxic injury. Special stains showed normal myelination for gestational age. Histologic brain sections, taken from autopsy cases of fetuses at the same gestational age, a week older and a week younger, were stained for comparison. Routine sections taken from other organs showed no pathologic changes. The postmortem 7DHC:cholesterol ratio was 42 µg ml: 3 mg/ml. Both 7-dehydrocholesterol and 8-dehydrocholesterol levels normally fall below 0.2 µg/ml. The normal range for cholesterol content in amniotic fluid is 8–36 mg/ml.
In view of an almost 4% carrier rate the homozygote frequency may be as high as 1/2,500 [Opitz et al., 2002]. However, the birth prevalence of affected infants is no higher than 1/15,000–1/20,000 [Bzdúch et al., 2000]. Thus, the prenatal mortality in the RSH syndrome may be close to 90%. Since in Western populations most autosomal recessive conditions occur as chance-isolated cases, a careful pathologic, biochemical and DNA study may be the only way to identify parents of lethally affected infants as carriers of the RSH syndrome with 25% recurrence risk.
Parents of children with RSH syndrome need to be aware of the availability of prenatal diagnosis in future pregnancies. Prenatal diagnosis of RSH syndrome requires a demonstration of an elevated 7DHC to cholesterol ratio in fetal tissue, or an elevated 7DHC level in amniotic fluid. The 7DHC to cholesterol ratio can be determined at 11–12 weeks of gestation using tissue obtained by chorionic villous sampling. When a low unconjugated estriol (uE3) level is detected as part of the maternal serum screening, RSH syndrome should be considered a diagnostic possibility. As cholesterol synthesis is decreased in fetuses with this syndrome, steroid hormone levels such as uE3 will be low as they are derived from cholesterol. It has also been demonstrated that mothers of affected infants excrete in their urine a characteristic combination of dehydroestriol (DHE3) and DHPT (dehydropregnanetriol) [Shackleton et al., 2001]. Diagnosis of RSH syndrome is important so that carriers can be identified and receive genetic counseling. Also, infants, children, and even some adults have shown improvement of symptoms with dietary supplementation of cholesterol. With the availability of DNA analysis, amniocentesis, urine for DHE3 and DHPT, and the use of ultrasonography to assess anomalies, a prenatal diagnosis of RSH syndrome with a prognostic prediction should be possible.
Historical Footnote
It was the perspicacious R. Brian Lowry, then still working in Vancouver, who pointed out to one of us (JMO) that the presumably classical Meckel syndrome infant CH, “sold” to an unsuspecting public in a detailed review on Meckel syndrome by Opitz and Howe [1969] most likely had the RSH syndrome, a suspicion confirmed by the distinguished pediatric pathologist Enid Gilbert-Barness (then still in Madison) on a review of the autopsy and histologic slides confirming presence of cytomegaly and large hyperchromatic nuclei in the pancreatic island cells characteristic of the RSH syndrome (Fig. 8).
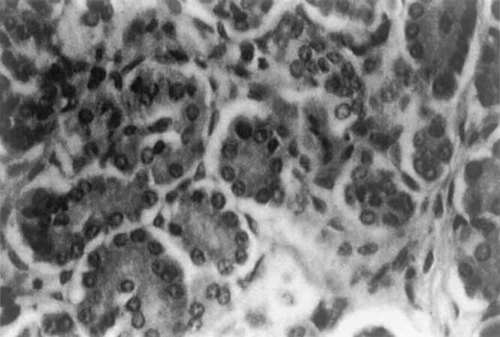
Section of pancreas of the infant described by Opitz and Howe [1969] prepared by Dr. E. Gilbert-Barness to show cytomegaly and large hyperchromatic nuclei in the islands of Langerhans; supporting evidence that this infant had the RSH rather than Meckel syndrome.
Thus, we emphasize that the RSH syndrome may be a relatively common cause of prenatal death, that its mostly chance-isolated occurrence in live- and stillborn infants puts a disproportionate onus of burden on prenatal detection and postmortem diagnosis subsequently allowing a substantial measure of prevention through DNA analysis of autopsy tissue and of lymphoblasts from parents, maternal serum triple analyte and urine metabolite screening, CVS, and amniocentesis. Ultrasonographers, clinicians, and pathologists must maintain a high index of suspicion because of the frequently atypical presentation of the condition which is now moving up on the list of causes of non-immune hydrops, at times even without toe 2/3 syndactyly. We strongly recommend RSH screening in all cases of idiopathic stillbirth, and that with the conversion to mass spectrometry, the RSH syndrome be included in the list of conditions screened in statewide newborn screening programs.
Acknowledgements
We are thankful to the P. family for most gracious permission to publish on their infants' condition, and to Dr. R. Brian Lowry and Dr. Enid Gilbert-Barness for pointing out the obvious concerning the “classic” case of Meckel syndrome who, in fact had the RSH syndrome, and to Nicole Wilson for expert document production.




