9q34.3 deletion syndrome in three unrelated children†
M. Iwakoshi and N. Okamoto contributed equally to this work.
Abstract
We described three unrelated children with cryptic 9q34.3 rearrangements and similar clinical manifestations: two with 9q34.3 terminal deletions and the other with an unbalanced translocation involving 9q34.3-qter monosomy and 6p25-pter trisomy. Common features among the three we studied and the other six patients with 9q34.3 deletions in the literature include microcephaly, mental retardation (MR), hypotonic, and epileptic seizures. Their facial characteristics included flat face, arched eyebrows, synophrys, hypertelorism, short nose, anteverted nostrils, carp mouth, protruding tongue, micrognathia, and pointed chin. Other frequent abnormalities were cardiac abnormalities, cryptorchidism or hypospadias, and abnormal toes. These findings are characteristic enough to be a clinically recognizable syndrome. © 2004 Wiley-Liss, Inc.
INTRODUCTION
Subtelomeric chromosomal aberrations are one of major causes in mental retardation (MR) with or without multiple anomalies. Advent of the first generation of subtelomere clones [National Institutes of Health and Institute of Molecular Medicine Collaboration, 1996] and an updated optimized set of clones [Knight et al., 2000] can facilitate analysis specific to subtelomeric chromosomal aberrations. A total of 20 studies focusing upon subtelomeric regions in 2,570 MR subjects identified 124 aberrations (4.8%) (a review by De Vries et al. [2003]). Besides mostly microscopically visible deletions including 4p− (Wolf–Hirschhorn), 5p− (cri du chat), 9p−, 13q−, and 18p− syndromes, submicroscopic deletions of 1p, 2q, and 22q were frequently observed [De Vries et al., 2003].
Here, we report on a 9q34.3 terminal deletion identified in three unrelated patients. Two of them were found in our on-going screenings of MR children by subtelomere-specific microarray comparative genomic hybridization (CGH) or FISH analysis using all-subtelomere probes. Another patient was suspected to have 9q subtelomeric deletion because of his clinical manifestations. They had clinical manifestations similar to but somewhat different from those in the previously reported patients.
PATIENT ASCERTAINMENT PROCEDURES
The three patients were derived from two ongoing screening programs for subtelomeric rearrangements in children with idiopathic MR with or without malformations. One, involving multi-subtelomeric FISH analysis using TelVysion™ (Vysis, Downer Grove, IL), identified Patient 1. Patient 3 was added to the program, prompted by the similarity of his phenotype to that of Patient 1. The other screening program used microarray CGH as described elsewhere [Fiegler et al., 2003]. Patient 2 was identified through the program. Briefly, DOP-PCR amplified clone DNA was spotted in quadruplicate onto solid activated glass slides (Motorola, Northbrook, IL) using a spotter, Stampman™ (Nippon Laser & Electronics Laboratory, Nagoya, Japan). The quadruplicated spots were printed twice to blocks A and B on the same slide. Subject DNA was labeled with Cy3 (Amersham Bioscience, Buckinghamshire, UK) and control DNA with Cy5 (Amersham Bioscience) (CGH1), and reverse labeling (patient's DNA with Cy5 and control DNA with Cy3) was also performed (CGH2). Labeled probe mixtures of CGH1 and CGH2 were simultaneously applied to blocks A and B, respectively. Slides were incubated at 37°C for 72 hr by gentle shaking, washed, scanned with GenePix 4000B (Axon Instruments, Inc., Union City, CA), and analyzed with GenePix Pro 4.0 software (Axon Instruments, Inc.). The intensity ratio between patient's and control DNA was calculated using ‘the ratio of means’ formula.
CLINICAL REPORTS
Patient 1
A 2-year-old girl. She was born to a 32-year-old G1P0 mother and a non-consanguineous 37-year-old father. Family history was unremarkable. The girl was delivered at 41 weeks of gestation with a birth weight of 3,030 g (−0.5 SD), length 45 cm (−2.5 SD), and OFC 32 cm (−1.5 SD). Anal atresia was operated at age 4 weeks. Developmental milestones were delayed: she lifted her head at age 8 months, sat alone at 20 months, and crawled at 2¼ years, but did not walk at 2⅔ years. At age 23 months, she developed febrile seizures. She was referred to us at age 8 months for developmental delay. Abnormalities included microcephaly, a round face, pointed chin, arched eyelashes with synophrys, down-slanted palpebral fissures, a short nose, flat nasal root, anteverted nostrils, open carp mouth, thick lower lip, protruding tongue, exudative otitis media, and hypotonia (Fig. 1, Tables I and II). She had a ventricular septal defect surgically corrected at age 11 months, pulmonary hypertension, hypothyroidism, left hydronephrosis, abnormal position of a left 4th toe, and clinodactyly of a right 2nd toe. Her DQ at age 2¼ years was estimated at 25. Brain MRI showed mild dilatation of the left ventricle. Now aged 2⅔ years, she measures 81.2 cm (−2.4 SD) and weighs 10.1 kg (−1.6 SD). She does not speak any meaningful words. GTG-banded chromosomes at the 550-band level were normal. Subtelomere FISH using all TelVysion probes showed a 9q34.3 terminal deletion, a finding confirmed by another 9q subtelomere probe (GS-135I17; 65 kb from the 9q telomere) [Knight et al., 2000] (Fig. 2a). Both her parents had normal chromosomes 9 by FISH using GS-135I17.
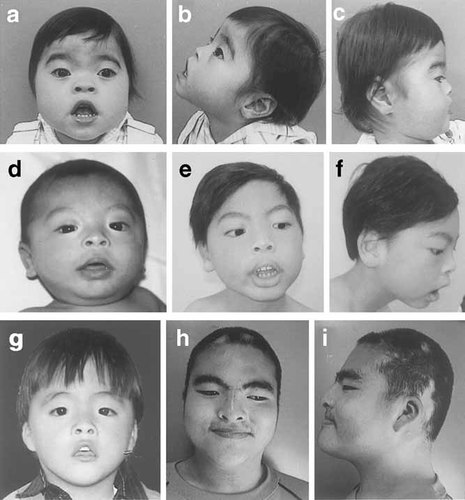
Patient 1 at age 2⅔ years (a, b, and c), Patient 2 at ages 5 months (d) and 7 years (e and f), and Patient 3 at ages 2 years (g) and 13 years (h and i).
| Present series |
Schimmenti et al., 1994 |
Ayyash et al., 1997 |
Dawson et al., 2002 |
Cormier-Daire et al., 2003 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 1 | Patient 2 | Patient 1a | Patient 2 | |||
| Karyotype | del(9)(q34.3) | del(9)(q34.3) | der(9)t(6;9) | del(9)(q34.3) | del(9)(q34.3) | der(9)t(5;9) | del(9)(q34.3) | del(9)(q34.3) | del(9)(q34.3) |
| de novo | de novo | (p25;q34.3)de novo | de novo | de novo | (q35.3;q34.3)pat | De novo | de novo | de novo | |
| Sex | F | M | M | M | M | F | F | M | M |
| Age (years) | 2 8/12 | 7 | 13 | 5 mo. | 5 mo. | 9 mo. | 6 | 9 | 22 mo. |
| Gestational age at birth (weeks) | 41 | 40 | 38 | 36 | 38 | 41 | 41 | Term | Term |
| Birth weight (g) | 3030 [−0.5 SD] | 3008 [−0.4 SD] | 2620 [−0.8 SD] | 2000 | 3010 [10%ile] | 3473 [50%ile] | 3410 | 3200 | 3920 |
| Birth length (cm) | 45 [−2.5 SD] | 48 [−1.0 SD] | 45.4 [−1.7 SD] | 49 [>10%ile] | 40 [25–50%ile] | 50 | 50 | ||
| Birth OFC (cm) | 32 [−1.5 SD] | 32 [−1.5 SD] | 32.6 [−0.8 SD] | 30.3 [<3%ile] | 31.5 [2%ile] | 34.5 | 33 | ||
| Present weight (kg) | 10.1 [−1.6 SD] | 18.4 [−1.5 SD] | 77.4 [+2.9 SD] | 4.1 [<5%ile] | 7.6 [10–25%ile] | 24 [80%ile] | 72 [>+6 SD] | 14.5 [>+2 SD] | |
| Present height (cm) | 81.2 [−2.4 SD] | 114 [−1.9 SD] | 162.9 [+0.5 SD] | 58 [<5%ile] | 68.5 [25-50%ile] | 108 [5–10%ile] | 144 [+2 SD] | 85 [normal] | |
| Present OFC (cm) | 43.5 [−2.8 SD] | 46.3 [−3.8 SD] | 55 [+0.5 SD] | 37 [<5%ile] | Microcephalic | 39.9 [2%ile] | 3%ile | 52.5 [−0.5 SD] | 46 [−2 SD] |
- a Also reported by Rio et al. [2002].
| Clinical Manifestation | Present series |
Schimmenti et al., 1994 |
Ayyash et al., 1997 |
Dawson et al., 2002 |
Cormier-Daire et al., 2003 |
Positive ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 1 | Patient 2 | Patient 1a | Patient 2 | ||||
| Brachycephaly | − | − | − | − | − | + | + | 2/7 | ||
| Flat face | + | + | + | − | + | + | + | + | 7/8 | |
| High forehead | − | + | − | + | + | + | 4/6 | |||
| Arched eyebrows | + | + | + | + | + | 5/5 | ||||
| Synophrys | + | − | + | − | + | + | + | 5/7 | ||
| Hypertelorism | + | + | + | + | + | + | + | + | + | 9/9 |
| Down slanted palpebral fissures | − | + | + | + | + | Up slanted− | Up slanted− | + | 5/8 | |
| Flat nasal root | + | − | − | − | − | + | + | 3/7 | ||
| Short nose | + | + | + | + | + | 5/5 | ||||
| Anteverted nostrils | + | + | + | + | + | + | 6/6 | |||
| Short philtrum | − | + | − | + | − | 2/5 | ||||
| Open carp mouth | + | + | + | + | + | 5/5 | ||||
| Thin upper lip | + | − | +/− | + | + | + | + | 6/7 | ||
| Thick lower lip | + | + | +/− | 3/3 | ||||||
| Protruding tongue | + | + | + | + | + | + | 6/6 | |||
| Micrognathia | + | + | − | + | + | 4/5 | ||||
| Pointed chin | + | + | + | + | 4/4 | |||||
| Prognathism | − | − | + | + | + | 3/5 | ||||
| Low set ears | − | + | − | + | + | 3/5 | ||||
| Malformed ears | − | − | + | + | + | 3/5 | ||||
| Exudative otitis media | + | − | − | 1/3 | ||||||
| Partial alopecia | + | + | + | 3/3 | ||||||
| Brown thin soft hair | + | + | + | 3/3 | ||||||
| Skeletal abnormality | − | − | − | + | 1/4 | |||||
| Short extremities | − | − | − | + | + | 2/5 | ||||
| Abnormal toes | + | − | Left poly-dactyly | + | + | Syndactyly (toes 2–3) | 5/6 | |||
| Cardiac anomaly | VSD | VSD | Heart murmur | SVT | VSD | Normal | VSD | ASD | 7/8 | |
| Asthma | − | + | + | 2/3 | ||||||
| Hydronephrosis | + | + | − | 2/3 | ||||||
| Cryporchidism | − | + | − | − | + | + | 4/6 | |||
| Hypospadias | − | − | + | − | + | + | 3/6 | |||
| Anal atresia | + | − | − | 1/3 | ||||||
| Obesity | − | − | + | − | − | − | + | + | 3/8 | |
| Hypothyroidism | + | − | − | + | 2/4 | |||||
| Hypotonia | + | + | + | + (Mild) | + | + (Infancy) | + | 7/7 | ||
| Epilepsy | + | + | − | Normal EEG | + | − | 3/6 | |||
| DQ/IQ (at age years) | DQ25 (2 3/12) | DQ < 25 (7) | DQ49 (3 7/12) DQ32 (13) | DQ 40 (9/12) | Moderate to Severe MR | DQ50 (9) | ||||
| Antistic behavior | + | − | +/− | + | 3/4 | |||||
- a Also reported by Rio et al. [2002].
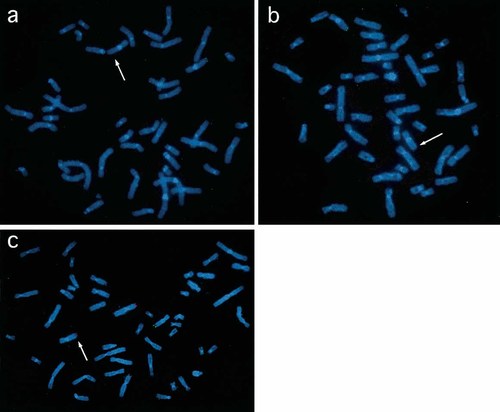
FISH studies of Patients 1 (a), 2 (b), and 3 (c). Green signal of 9q34.3 subtelomere probe was deleted in all patients (arrows). Red signal of 6p25 subtelomere probe was translocated to the derivative chromosome 9 in Patient 3 (c).
Patient 2
A 7-year-old boy. He was born to a 23-year-old G1P0 mother and a non-consanguineous 24-year-old father. His younger brother and sister are both healthy. He was delivered at 40 weeks of gestation with a birth weight 3,008 g (−0.4 SD), length 48 cm (−1.0 SD), and OFC 32 cm (−1.2 SD). Developmental milestones were delayed: he lifted his head at age 12 months, sat at 7 years, but could not crawl or walk at 7 years. At age 2 years, he developed generalized seizures, controllable with medication. He was referred to us as a neonate. Abnormalities noted included microcephaly; a round face; pointed chin; arched eyelashes; down-slanted palpebral fissures; a short nose with anteverted nostrils; open carp mouth; thick lower lip; protruding tongue; and hypotonia (Fig. 1, Tables I and II). He had a ventricular septal defect subsequently operated at age 9 months, pulmonary hypertension, and bilateral hydronephrosis. Brain MRI was normal. Now aged 7 years, his height is 114 cm (−1.9 SD) and weight 18.4 kg (−1.5 SD). He speaks no meaningful words, and his DQ is <25. GTG-banded chromosomes were a normal with a 46,XY karyotype. Microarray CGH analysis indicated a 9q subtelomeric deletion without abnormalities in other subtelomeric regions. FISH using GS-135I17 as a probe supported the 9q subtelomeric deletion in this patient (Fig. 2b), but no such abnormalities in his parents.
Patient 3
The patient, a boy, was born at 38 weeks of gestation as a second child to a 26-year-old G2P1 mother and a non-consanguineous 34-year-old father. Family history was unremarkable. Birth weight was 2,620 g (−0.8 SD), length 45.4 cm (−1.7 SD), and OFC 32.6 cm (−0.8 SD). Developmental milestones were delayed: he lifted his head at age 6 months, sat alone at 12 months, and walked without support at 2⅓ years. He was referred to us at age 6 months. Abnormalities noted were a round face, pointed chin, arch-shaped eyelashes, down-slanted palpebral fissures, a short nose with anteverted nostrils, open carp mouth, thick lower lip, protruding tongue, polysyndactyly between left toes 5 and 6, and hypotonia (Fig. 1, Tables I and II). Bilateral cryptorchidism was surgically repaired at age 1 year. He spoke one-word sentences at age 23 months and two-word sentences at 5 years. His DQ at age 3 7/12 years was 49 and at age 13 years was 32. Brain MRI at age 5 years indicated mild dilatation of the left ventricle. Now aged 13 years, his height is 162.9 cm (+0.5 SD) and weight 77.4 kg (+2.9 SD). The change of his facial appearance is obvious. For example, his nose is no longer short, and his mouth is closed. Routine chromosome analysis revealed a normal 46,XY karyotype. FISH study using all TelVysion probes showed replacement of a 9qter signal, by a 6pter signal. FISH using GS-135I17 validated a 9q34.3 deletion, and FISH with the 6p subtelomere probe (GS-62I11) clearly demonstrated a signal on the der(9) (Fig. 2c). FISH analysis of his parents using subtelomere probes for 6p and 9q showed no abnormality.
DISCUSSION
We described three unrelated children with cryptic 9q34.3 rearrangements and similar clinical manifestations: Patients 1 and 2 with 9q34.3 terminal deletions, and Patient 3 with an unbalanced translocation involving 9q34.3-qter monosomy and 6p25-pter trisomy. Partial trisomy for 6p25-pter has not been reported in the literature, but several instances of 6p23-pter trisomy have been described [Rothlisberger et al., 1999; Schinzel, 2001]. The clinical manifestations in individuals with 6p23-pter trisomy included intrauterine growth retardation, short stature, microcephaly, prominent forehead, low-set and malformed ears, blepharoptosis, small mouth with thin upper lip, and cardiac defects. Most of these manifestations overlapped with those of Patients 1 and 2. Non-overlapping features such as blepharoptosis and small mouth were not present in Patient 3. In view of these findings, the phenotypic effect of 6p25-pter trisomy in Patient 3 was assumed to be minimal.
Review of the literature uncovered 12 individuals with deletions or unbalanced translocations involving 9q34.3 monosomy. In six of them, descriptions of clinical manifestations were too brief, all being identified in the course of systematic screening for subtelomeric rearrangements in subjects with idiopathic MR [Breen et al., 1999; Knight et al., 1999; De Vries et al., 2001; Fauth et al., 2001; Rossi et al., 2001; Anderlid et al., 2002]. The clinical manifestations in the other six individuals have been described in sufficient detail. These six individuals were analyzed for clinical manifestations, together with the three children we described (Tables I and II).
The nine subjects included six males and three females, raging in age from 5 months to 13 years (Table I). They consisted of seven children with 9q34.3 deletions and two with unbalanced reciprocal translocations. Of these, three were chromosomally normal with conventional G-banding analysis [present series]; two were normal on conventional G-banding analysis but were found to have a deletion on repeat analysis [Schimmenti et al., 1994; Ayyash et al., 1997]; another two were normal on both conventional and high-resolution banding analysis [Cormier-Daire et al., 2003]; two were normal on 550 G-banding analysis [Dawson et al., 2002]. In view of these findings, it is likely that the size of 9qter deletion differed among these individuals. Seven of the nine children were microcephalic, all five children 2 years or older were mentally retarded, seven were hypotonic, and three developed epileptic seizures. Their facial characteristics included flat face (7/8), arched eyebrows (5/5), synophrys (5/7), hypertelorism (9/9), short nose (5/5), anteverted nostrils (6/6), carp mouth (5/5), protruding tongue (6/7), micrognathia (4/5), and pointed chin (4/4) (Table II). Other frequent abnormalities were cardiac abnormalities (7/8), cryptorchidism or hypospadias (4/6), and abnormal toes (5/6). These findings are characteristic enough to be clinically recognizable syndrome. All three children we described had light-colored soft hair in early childhood and partial alopecia, and two of the three children developed asthma and hydronephrosis. These findings have not described in other individuals with 9ter deletions.
Ayyash et al. [1997] attempted to establish a clinically recognizable phenotype, based on two patients with 9q32-q34 deletion and another two patients with 9q34.3 deletion. The latter two patients [Schimmenti et al., 1994; Ayyash et al., 1997] are included in Tables I and II. Since their 9qter deletion was detectable on conventional G-banding analysis, the extent of their deletion is assumed to be larger than those unrecognizable on conventional or high-resolution banding analysis. Cormier-Daire et al. [2003] observed in two unrelated boys with de novo deletion 9q34, obesity, sleep disturbances, characteristic facial features, genital anomalies, and other abnormalities (Tables I and II). Patient 1 in their report was detected in a systematic screening for subtelomeric rearrangements in subjects with idiopathic MR, while Patient 2 was studied in view of his clinical similarities to Patient 1. Of the nine children with 9q34.3 deletion we analyzed, only three had obesity: the two boys reported by Cormier-Daire et al. [2003] and a boy (Patient 3) we described. Obesity is thus likely to be infrequent in individuals with 9qter deletion, but warrants screening when present in combination with idiopathic MR.
In conclusion, we described two boys with 9q34.3 terminal deletions and a boy with an unbalanced translocation with der(9)t(6;9)(p25;q34.3). The clinical manifestations in the three children we described and in another six children reported were analyzed. It was concluded that they constitute a clinically recognizable phenotype.




