Exclusion of candidate genes in a family with arterial tortuosity syndrome
Abstract
Arterial tortuosity syndrome (ATS) is a rare hereditary disorder with variable clinical presentation including tortuosity and elongation of the major arteries, often associated with pulmonary artery stenosis, pulmonary hypertension, and skin and joint laxity, suggestive of a connective tissue disorder. ATS is transmitted in an autosomal recessive mode, but the causal gene is unknown. We report an Italian pedigree with three inbred families in which five patients show signs of ATS. In particular, four adult patients present arterial tortuosity and elongation of the main arteries. Two of these patients, with the most severe degree of arterial tortuosity, also show severe peripheral stenosis of the main pulmonary artery. The fifth young patient shows a severe pulmonary valve stenosis in the absence of arterial tortuosity. All patients show signs of Ehlers–Danlos syndrome (EDS): soft skin with abundant subcutaneous tissue and joint laxity, hernias, and disorganization of the extracellular matrix (ECM) of fibronectin (FN) and of actin microfilaments in cultured skin fibroblasts. Linkage analysis of the genes involved in EDS and other connective tissue disorders, excluded COL1A1, COL1A2, COL2A1, COL3A1, COL5A1, COL5A2, COL5A3, COL6A1, COL6A2, ADAMTS2, ELN, FN1, TNXA, and TNXB as candidate genes in the family under study, thus indicating that ATS is a distinct clinical and molecular entity. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Arterial tortuosity sindrome (ATS [MIM 208050]) is a rare disorder characterized by tortuosity and elongation of the major arteries and often associated with pulmonary stenosis, pulmonary hypertension, and aneurysms [Beuren et al., 1969; Lees et al., 1969; Adès et al., 1996; Pletcher et al., 1996; Al Fadley et al., 2000; Franceschini et al., 2000; Rivera et al., 2000]. These arterial alterations result in cardiac findings such as ventricular hypertrophy, dilatation and obliteration, mitral and tricuspidal valve alterations, and systolic murmur. ATS patients also display skin laxity, joint laxity, contractures and dislocations, arachnodactyly, inguinal hernias and facial features, including micrognathia, frontal bossing, downslanted palpebrae, hypertelorism, keratoconus, beaked nose, and high-arched palate [Lees et al., 1969; Pletcher et al., 1996; Franceschini et al., 2000]. These clinical signs suggest that ATS is a connective tissue disorder. In particular, ATS shares several clinical skin-joint-vasculature signs with Ehlers–Danlos syndrome (EDS), a heterogeneous group of disorders in which alterations in several genes, such as types I, III, and V collagens, collagen-processing enzymes and tenascin X, have been reported [Steinmann et al., 1993; Pope and Burrows, 1997; Michalickova et al., 1998; Colige et al., 1999; Bouma et al., 2001; Mao and Bristow, 2001]. The biochemical and histological analysis of types I and III collagens synthesized by cultured skin fibroblasts, derived from ATS patients, has not shown alterations in the levels of these proteins [Adès et al., 1996; Pletcher et al., 1996; Franceschini et al., 2000]. We have reported that in vitro cultured skin fibroblasts, derived from different EDS types, independently from their molecular defect, show a defective extracellular matrix (ECM) of fibronectin (FN) and disorganized actin cytosckeleton [Moro et al., 1994; Zoppi et al., 1998]. ATS patients also show alterations present in genetic disorders of the cardiovascular system such as supravalvular aortic stenosis and Williams–Beuren syndrome, due to elastin gene (ELN) alterations [Li et al., 1997; Towbin et al., 1999; Milewicz et al., 2000]. Histological findings have shown disruption of elastic fibers of the arterial media and fragmentation of the internal elastic membrane in an ATS patient [Pletcher et al., 1996].
In the limited number of family cases so far reported, ATS is transmitted in an autosomal recessive mode, but the causal gene is not known [Pletcher et al., 1996; Al Fadley et al., 2000; Franceschini et al., 2000].
We report an Italian inbred pedigree with five affected ATS patients with variable clinical presentation and the exclusion, by linkage analysis, of genes known to be involved in EDS and in other connective tissue disorders.
MATERIALS AND METHODS
Skin Fibroblast Cultures and Indirect Immunofluorescence Analysis
Skin fibroblast cultures were established in our laboratory from arm biopsies from all the fourth-generation subjects, their parents (Fig. 1) and a control donor. All cell strains, used at the 4th in vitro passages, were grown in vitro at 37° C in modified Eagle's medium (Invitrogen-Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen-Life Technologies), 100 μg/ml penicillin and 100 μg/ml streptomycin. Informed consent was obtained from all the members of the ATS family analyzed.
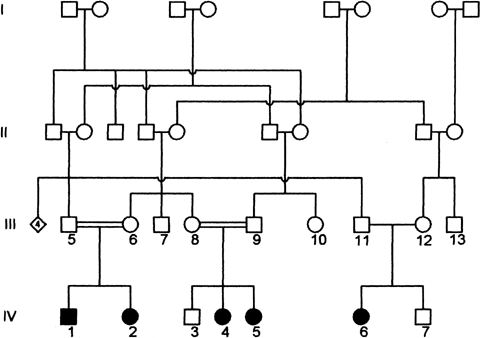
Four-generation arterial tortuosity syndrome (ATS) family pedigree. Clinical and genetic analyses were carried out in individuals from the third and the fourth generation.
The study of FN was performed by IF on control and ATS fibroblasts grown in modified Eagle's medium supplemented with 10% FBS. For this purpose, 1.5 × 105 cells of each strain were seeded on 22 × 22 mm glass coverslides and grown at 37°C in a 5% CO2 incubator for 48 hr. The cells were fixed in 3% paraformaldehyde and 60 mM sucrose for 5 min, permeabilized in 0.5% (v/v) Triton X-100 for 90 sec, washed 2× in PBS, and 0.15 M glycine for 3 min and immunoreacted with anti-FN polyclonal antibody (1:100 in diluting buffer containing 0.3% BSA and 0.1% NaN3 in PBS) (Sigma, St. Louis, MO). All cells were washed 3 × 5 min in PBS and reacted with rodhamine-conjugated goat anti-rabbit IgG (Calbiochem Novabiochem, San Diego, CA) (10 μg/ml), as secondary antibodies, for 45 min at RT. To analyze actin distribution the fibroblast strains were reacted for 30 min with 1 μg/ml FITC–phalloidin (Sigma, St. Louis, MO) and washed three times with PBS. The coverslides were mounted on glass slides in 1:1 PBS/glycerol solution; the IF signals were acquired by a CCD black and white TV camera (SensiCam-PCO Computer Optics GmbH, Germany) mounted on a Zeiss Axiovert fluorescence microscope and acquired by an Image Pro Plus program (Media Cybernetics, Silver Spring, MD).
DNA Analysis
DNA was purified from cultured fibroblasts using a standard method. The candidate genes were analyzed with the following intragenic markers: COL1A1 RsaI RFLP [Rose et al., 1991], COL1A2 dinucleotide repeat [Chi et al., 1992], COL2A1 VNTR [Priestley et al., 1990], COL3A1 dinucleotide repeat [Lee et al., 1991], COL5A1 [Greenspan et al., 1995], COL6A2 dinucleotide repeat [Comeglio et al., 1996], ADAMTS2 D5S1382 tetranucleotide repeat (Human Genome Browser), ELN tetranucleotide repeat [Urbán et al., 1997], FN1 MspI RFLP [Gardella et al., 1993], TNXA and TNXB Bsh1236I RFLP [Weissensteiner and Lanchbury, 1997]. The flanking D19S413 dinucleotide repeat was used for the analysis of COL5A3 [Imamura et al., 2000]. DNA was amplified with specific primers and digested with the appropriate restriction enzyme or analyzed on an ABI3100 capillary-sequencer by Genescan software (Applied Biosystems, Foster City, CA).
CLINICAL REPORT
Family Pedigree
Five patients with ATS are present in the four-generation ATS pedigree (Fig. 1). The patients belong to three different inbred familiar nuclei originating from the same area in Sicily (Italy). Four patients (IV:1 and IV:2; IV:3 and IV:4) were born from first cousins parents. Patient IV:6 was born from parents who, to their knowledge, are not consanguineous.
Patients IV:1 and IV:2
Patients IV:1 and IV:2 (Fig. 2) are 21- and 16-year-old siblings showing, as major alterations, tortuosity, dilatation, and elongation of the main arteries and an increase in carotid bifurcation intima-media thickness (IMT) (1.1 mm), associated with vomiting, failure to thrive, dyspnea, diaphoresis, arm and abdominal pain, and fainting (Fig. 3, Table I). Both patients also suffered recurrent fever, bronchitis, and pneumoniae (Table I). Patient IV:1 suffered a cardiac arrest at delivery. Patient IV:2 presented right arm paresthesia, aneurysm of the interatrial septum, and light aortic regurgitation. Connective tissues abnormalities were present in both patients: skin and joint laxity, hernias, and soft nasal cartilage (Table II). They also showed hypothyroidism with post-puberal onset. Lymphocyte chromosomes were normal. Concerning the prognosis of these patients, the evolution of the angiographic features is not known since only few observational and not conclusive studies on ATS patients are available [Pletcher et al., 1996; Al Fadley et al., 2000; Franceschini et al., 2000].
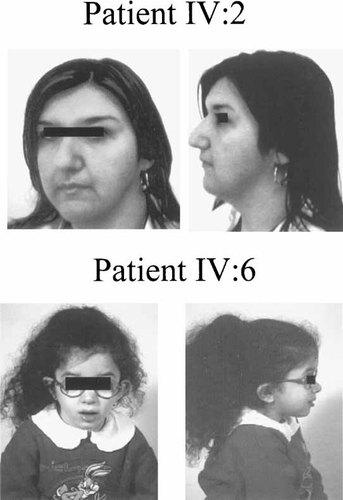
Frontal and lateral photographs of patients IV:2 and IV:6. Patient IV:2 shows the features observed also in patients IV:1, IV:3, and IV:4.
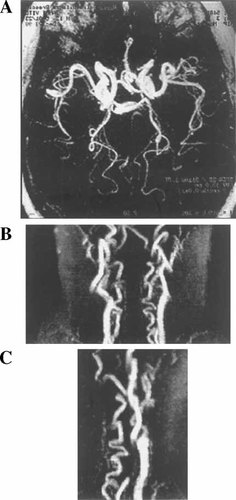
Tortuosity of cranial arteries and supraaortic vessels detected in patient IV:1 by MR angiography. A: Circle of Willis, axial view. B: Supraaortic vessels, AP view. C: Supraaortic vessels, lateral view. Patient IV:2 showed the same pattern.
| Case | Vomiting, failure to thrive, headache | Tortuosity, dilatation elongation site(s) | MPAa peripheral stenosis | Ventricular hypertrophy and remodeling | Systolic murmur | Palpitations, dyspnea, diaphoresis, fever | Bronchitis, pneumonia | MPA valve stenosis/replacement | Other findings |
|---|---|---|---|---|---|---|---|---|---|
| IV:1 | + | Aorta, pulmonary arteries, carotid, subclavian, vertebral, abdominal aorta, cranial | − | − | − | + | + | − | Increased IMTc, pallor crises, abdominal pain |
| IV:2 | + | Aorta, pulmonary arteries, carotid, subclavian, vertebral, abdominal aorta, cranial | − | − | − | + | + | − | Increased IMT, cardiac arrest at delivery, faintings, adbominal pain, right arm paresthesia |
| IV:4 | + | Aortic arch, carotid, subclavian, vertebral | + SPAPb 105 mmHg | ++ (right) | + | ++ | + | − | Increased IMT, adbominal pain |
| IV:5 | + | Aortic arch, carotid, subclavian, vertebral | + SPAP 90 mmHg | ++ (right) | ++ | ++ | + | − | Increased IMT |
| IV:6 | + | − | − | − | − | + | + | + | Increased IMT, right arm paresthesia |
- a MPA, main pulmonary artery.
- b SPAP, systolic pulmonary artery pressure.
- c Carotids intima-media thickness (IMT).
| Case | Skin findings | Joints findings | Hernia | Other findings | Fibroblasts findings |
|---|---|---|---|---|---|
| IV:1 | Soft, abundant subcutaneous tissue, ecchymosis | Laxity | Two inguinal at delivery, one surgically removed at 4 months | Soft nasal cartilage, fixity of facial expression, short fingers, hypothyroidism | FN–ECM reduction and disorganization, actin microfilaments disorganization |
| IV:2 | Soft, abundant subcutaneous tissue | Laxity | One inguinal | Soft nasal cartilage, keratoconus, intestine elongation, hypothyroidism | FN–ECM reduction and disorganization, actin microfilaments disorganization |
| IV:4 | Laxity | Hypermobile | — | Soft nasal cartilage, micrognathia | FN–ECM reduction and disorganization, actin microfilaments disorganization |
| IV:5 | Laxity | Hypermobile | — | Soft nasal cartilage, micrognathia, keratoconus | FN–ECM reduction and disorganization, actin microfilaments disorganization |
| IV:6 | Ecchymosis | Laxity | One gastric (cardias) at birth | Frontal bossing, soft nasal cartilage, micrognathia, myopia, prominent ears | FN–ECM reduction and disorganization |
Carotid ultrasonography analysis performed on the patients' parents, who were free of clinical signs, showed a mild increase in carotid IMT (1.0 mm). In the mother of the patient (III:6) moderate aortic insufficiency, consequent to prolapse of the left non-coronaric cusp, and a moderate hypertrophy of the left ventricle were also observed by 2D-Echo, together with moderate mitral and tricuspid valve regurgitation.
Patients IV:4 and IV:5
These patients aged 21 and 19 year, respectively, show, as major signs, tortuosity of the main systemic arteries and severe peripheral stenosis of the pulmonary tree (Fig. 4) leading to high systolic pulmonary artery pressure (SPAP) and to severe ventricular hypertrophy and cardiac remodelling (Fig. 5, Table I). Doppler estimation of tricuspidal ventricular regurgitation showed that SPAP in patient IV:4 was 105 mmHg and in her sister 90 mmHg (Table I). Both patients showed carotid IM thickening (1.06–1.25 mm) and moderate aortic insufficiency, consequent to prolapse of the left non-coronaric cusp. Skin and joint abnormalities were also present in both patients (Table II). Lymphocyte chromosomes were normal. The prognosis of the pulmonary hypertension is usually unfavorable but it can vary from case to case. The diagnosis of pulmonary hypertension in these ATS patients gives information useful in avoiding a rapid progression of the disease which could be due for example to pregnancy in the affected females.
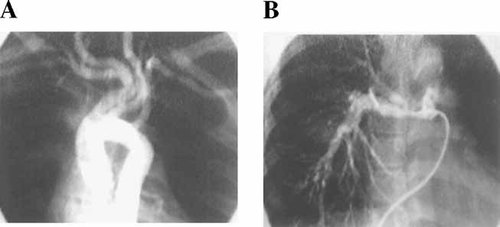
A: Aortic and carotid artery angiography of patient IV:5 showing marked tortuosity and dilatation of the carotid arterial system. B: Pulmonary artery angiography showing pruning of distal vessels. Angiography of patient IV:4 showed comparable alterations.
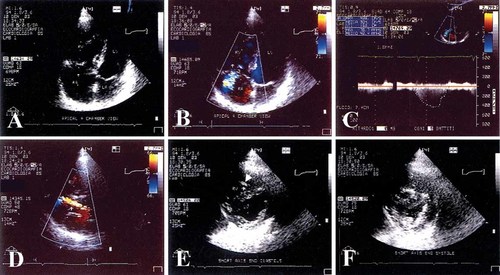
Echo-Doppler evaluation of patient IV:4. A: Echocardiographic apical four-chamber view (4CH) in end diastole. The left ventricle (LV), the left atrium (LA), the right ventricle (RV), and the right atrium (RA) are shown. The RV is enlarged, while LV dimensions are reduced. B: 4CH view, medium (+++) tricuspid regurgitant flow from the RV to the RA. C: Continuous doppler of the tricuspidal flow velocity from the 4CH view, the estimated systolic arterial pressure is 90 mmHg. D: Echocardiographic long axis view, a moderate (++) aortic regurgitant flow from the aorta to the LV is displayed for a moderate prolapse of the left non-coronaric aortic leaflet. E, F: Short axis of the LV and RV, evaluated at end diastole and at end systole, respectively. LV remodeling, consequent to RV dilatation, is shown: LV dimensions are reduced and the inter-ventricular septum is shifted towards the LV. Moderator band hypertrophy in the RV is also evident (arrow). Similar findings are observed in patient IV:5.
Carotid ultrasound performed on the patients' parents, and on their young unaffected brother (IV:3), revealed a mild increase in carotid IMT. Cardiac Doppler analysis of the patients' mother (III:8) revealed prolapse of the aortic cusp and aortic insufficiency, right ventricle hypertrophy, and moderate pulmonary hypertension (SPAP 42 mmHg). The patients' brother (IV:3) showed a moderate aortic insufficiency consequent to prolapse of the left non-coronaric cusp.
Patient IV:6
Patient IV:6 (Fig. 2) is a 6-year-old girl showing since birth severe pulmonary valve stenosis (Fig. 6), failure to thrive, headache, ventricular hypertrophy, systolic murmur, palpitations, dyspnea, and diaphoresis (Table I). At 2 and 4 years, the pulmonary stenotic valve was dilatated and at the age of 6 the valve was surgically removed and replaced with a bioprosthesis. At present, the patient shows mild valvular diastolic regurgitation. Arterial tortuosity is absent, although quantitative carotid ultrasound analysis showed increased IMT (0.8 mm) (Table I). Other signs are growth retardation, recurrent fever, bronchitis, and pneumonia (Table I), and frontal bossing, micrognathia, soft nasal cartilage, joint laxity, and gastric hernia at birth (Table II). This patient is the only one reported in the family pedigree showing a low growth centile (below 1%). Lymphocyte chromosomes are normal. Prognosis of the untreated severe pulmonary artery valve stenosis is short-term unfavorable and is greatly improved after surgical replacement of the valve. In the patient's mother (III:5), who is without clinical signs, carotid ultrasound highlighted a mild increase in carotid IMT, and slight aortic regurgitation due to elongation of the left non-coronaric cusps. The patient's 4-year-old brother (IV:7), without clinical signs, showed a mild increase in carotid IMT (0.7 mm) and mild tortuosity of internal and external carotids on both sides.
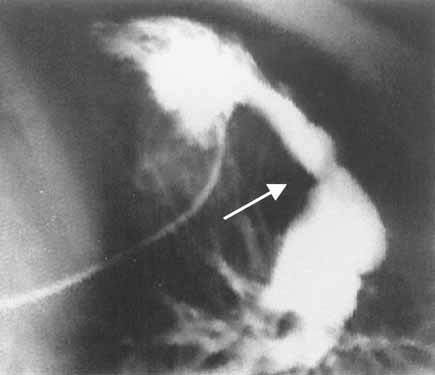
Cardiac catheterization of patient VI:6 at the age of 4 years showing severe stenosis of the pulmonary artery valve (arrows), hypertrophic right ventriculum, and dilatation of the pulmonary artery.
RESULTS
Skin Fibroblast Analysis
Immunofluorescence analysis of the ECM of FN organized by all ATS patients skin fibroblasts, performed with an anti-human-FN polyclonal antibody, showed the reduction and disassembly of the structure, as compared to skin fibroblasts derived from an unaffected donor (Fig. 7). Skin fibroblasts derived from the patients' parents organized a rich FN–ECM meshwork, although the appearance was less fibrillar than observed in control fibroblasts (data not shown). The immunofluorescence analysis with FITC–phalloidin of the actin microfilaments in all ATS patients except for patient IV:6, showed a varying degree of disorganization of these structures (Fig. 7). On the contrary, fibroblast perturbation of actin cytoskeleton organization was not observed in the patients' parents (data not shown).
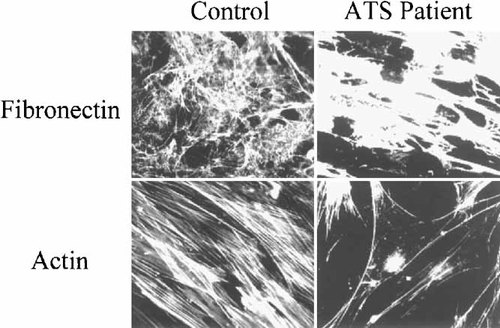
Immunofluorescence microscopy of skin fibroblasts derived from a control donor and from ATS patient IV:1 reacted with an anti-human fibronectin (FN) polyclonal antibody and with FITC–phalloidin. Disorganization of the extracellular matrix (ECM) of FN and of the actin microfilaments can be observed in ATS skin fibroblasts.
DNA Analysis
Linkage analysis for the candidate genes was performed in the ATS family members (third and fourth generation) using intragenic or closely linked markers. The genes analyzed encode for: collagen of different types (COL1A1, COL1A2, COL2A1, COL3A1, COL5A1, COL5A3) and a collagen-processing enzyme (ADAMTS2), which have been reported to be involved in EDS [Pope and Burrows, 1997; Mao and Bristow, 2001; Tang, 2001]; tenascin X (TNXA/B), also involved in EDS [Mao and Bristow, 2001]; fibronectin (FN1), which is reduced in cultured EDS fibroblasts [Moro et al., 1994; Zoppi et al., 1998]; COL6A2, altered in Bethlem myopathy [Jöbsis et al., 1999]; and elastin (ELN), involved in cutis laxa, supravalvular aortic stenosis, and Williams–Beuren syndrome [Towbin et al., 1999; Milewicz et al., 2000].
As shown in Table III, homozygosity was not detected in all affected ATS patients for any of the genes tested. The results excluded these genes from the ATS etiology in this family. Moreover, since COL5A2 is close to COL3A1 gene on chromosome 2q32.2, and COL6A1 is linked to COL6A2, on chromosome 21q22.3, our data also exclude COL5A2 and COL6A1 as candidate genes in this family.
| Gene | Chromosome | Marker | IV:1 ▪ | IV:2 ● | IV:3 □ | IV:4 ● | IV:5 ● | IV:6 ● | IV:7 □ |
|---|---|---|---|---|---|---|---|---|---|
| COL1A1 | 17q21.33 | Intragenic RsaI RFLP | +/+a | +/+ | +/− | +/− | +/− | +/+ | +/+ |
| COL1A2 | 7q21.3 | Intragenic dinucleotide repeat | 2/5b | 5/5 | 2/7 | 5/7 | 2/5 | 7/7 | 5/7 |
| COL2A1 | 12q13.11 | Intragenic VNTR | 4/4 | 3/4 | 1/2 | 2/4 | 2/4 | 4/4 | 4/4 |
| COL3A1 | 2q32.2 | Intragenic dinucleotide repeat | 3/6 | 3/5 | 7/7 | 3/6 | 7/7 | 3/5 | 3/5 |
| COL5A1 | 9q34.3 | Intragenic dinucleotide repeat | 4/5 | 5/5 | 4/5 | 5/5 | 5/5 | 4/5 | 4/4 |
| COL5A3 | 19p13.2 | D19S413 dinucleotide repeat | 8/9 | 8/9 | 8/10 | 8/10 | 3/10 | 3/8 | 3/8 |
| COL6A2 | 21q22.3 | Intragenic dinucleotide repeat | 6/7 | 3/7 | NDc | 3/7 | 3/7 | 6/7 | 6/7 |
| ADAMTS2 | 5q35.3 | Intragenic tetranucleotide repeat | 2/2 | 2/2 | 1/2 | 1/2 | 1/2 | 1/2 | 2/2 |
| ELN | 7q11.23 | Intragenic tetranucleotide repeat | 2/13 | 12/12 | 12/13 | 12/13 | 12/12 | 12/13 | 12/12 |
| FN1 | 2q35 | Intragenic MspI RFLP | +/+ | +/− | +/− | +/− | +/− | +/− | +/− |
| TNXA | 6p21.33 | Intragenic Bsh1236I RFLP | +/+ | +/− | +/− | +/− | −/− | +/− | +/+ |
| TNXB | +/+ | +/− | +/− | +/− | +/− | +/− | +/+ |
- a Presence (+) or absence (−) of the restriction site.
- b Alleles are reported as in GDB; the new alleles are numbered consecutively to the GDB reported alleles.
- c Not determined.
DISCUSSION
ATS is an uncommon disorder characterized by generalized tortuosity, elongation, and stenosis of the major arteries with an autosomal recessive mode of transmission but unknown etiology [Beuren et al., 1969; Lees et al., 1969; Adès et al., 1996; Pletcher et al., 1996; Al Fadley et al., 2000; Franceschini et al., 2000; Rivera et al., 2000].
We describe a four-generation pedigree with three inbred families with four adult patients showing as major signs systemic arterial tortuosity, severe pulmonary artery tree stenosis, and variable cutaneous, facial and joint manifestations. A fifth young patient in this family shows congenital pulmonary valve stenosis, cutaneous, joint and facial manifestations, in the absence of major arterial involvement. In these patients gastric and inguinal hernias, intestine elongation, and keratoconus were occasionally found. These patients came to our attention due to a diagnosis of EDS; after clinical and instrumental investigations, we could ascribe to these patients an ATS phenotype with variable manifestation. Due to the inbred family pedigree, we hypothesized a recessive mode of inheritance of the gene responsible for the disease in this family. Indeed, patients IV:1 and IV:2, as well as patients IV:4 and VI:5, were born from first cousin parents. Moreover, their mothers were two sisters and their fathers were first cousins. Patient IV:6, who did not have consanguineous parents, belongs to the same pedigree since her father is the brother of the father of patients IV:1 and IV:2 and her mother is the first cousin of the mothers of the other patients. Although the parents of IV:6 patients are not consanguineous, we cannot exclude the presence of common ancestors in previous generations, due to their common geographical origin.
Carotid ultrasonography and echocardiography in the patients' parents and brothers, who are without clinical signs, showed a mild to moderate increase in carotid IMT and/or moderate aortic insufficiency due to prolapse of the left non-coronaric cusps. Moreover, the mother of patients IV:4 and IV:5 showed moderate pulmonary hypertension. The presence of these minimal cardiovascular signs suggests that these individuals could be heterozygous carriers of ATS.
In ATS, interfamilial and intrafamilial variability has been reported [Beuren et al., 1969; Lees et al., 1969; Adès et al., 1996; Pletcher et al., 1996; Al Fadley et al., 2000; Franceschini et al., 2000; Rivera et al., 2000]. Variable stigmata are present also in our ATS family. Patient IV:6 showed the greatest differences compared to the other ATS patients, since she only presented stenosis of the pulmonary valve, without detectable arterial tortuosity. Although arterial tortuosity was not present in this young patient, an alteration of the carotid artery thickness was detected. This minimal arterial involvement in childhood suggests a possible evolution based on hemodynamic reasons. In general, the other four patients showed a similar phenotype, which was more similar within the same sibship. For example, in sisters IV:4 and IV:5 the arterial pulmonary tree was more seriously altered than in their cousins, patients IV:1 and IV:2. This intrafamilial variability could be due to their different genetic background. However, since patient IV:6 was the only one for whom consanguineity was not ascertained, we cannot exclude that here more than one gene might be involved in the disease.
The analysis of cultured skin fibroblasts derived from the ATS-affected patients showed alteration of both the extracellular network of FN and the cytoplasmic actin microfilaments. Only in patient IV:6 was the cytoskeleton of actin not disturbed. These in vitro data are in agreement with the connective tissue alterations observed in these patients and suggest that the gene involved in ATS in this family participates in the organization and/or maintenance of the ECM. Similar FN–ECM patterns have been observed in several strains of in vitro cultured skin fibroblasts derived from EDS patients with different molecular defects, but not in cells derived from patients affected by other connective tissue disorders [Moro et al., 1994; Zoppi et al., 1998].
Since there are as yet no reports of DNA molecular investigations in ATS patients, we analyzed several candidate genes in the ATS family. On the basis of their inbreeding, strongly suggesting a recessive mode of transmission of the disease, we performed DNA linkage analysis by the homozygosity approach. We considered as possible disease genes those involved in EDS and other connective tissue disorders sharing clinical signs with ATS, particularly, with vascular involvement (COL1A1, COL1A2, COL2A1, COL3A1, COL5A1, COL5A2, COL5A3, COL6A1, COL6A2, ADAMTS2, ELN, FN1, TNXA, TNXB). This analysis, performed using intragenic or closely linked markers, did not show homozygosity for any of the genes studied, since at least one patient was heterozygous for each gene marker analyzed. In particular, heterozygosity for each gene was detected in patients having parents with ascertained consanguineity. Therefore, our findings exclude these as candidate genes in this ATS family. In particular, our analysis excludes COL3A1 gene, which is notoriously altered in the vascular type IV EDS [De Paepe, 1994; Liu et al., 1997; Mao and Bristow, 2001]. Type V collagen genes responsible for type I EDS variants, occasionally with cardiovascular involvement, are also excluded [Steinmann et al., 1993]. The results obtained at DNA level, excluding the involvement of types I and III collagens from ATS, are in agreement with previous biochemical and histological findings in ATS patients [Adès et al., 1996; Pletcher et al., 1996; Franceschini et al., 2000]. Our data also excluded ELN, a strong candidate in ATS, since histological findings have reported disruption of internal arterial elastic membrane in ATS patients [Pletcher et al., 1996] and elastin is the main ECM component of the arterial wall and an essential determinant of arteries morphogenesis [Li et al., 1998; Brooke et al., 2003]. Point mutations in ELN lead to supravalvular aortic stenosis, a generalized arteriopathy with aortic involvement and pulmonary stenosis as the main alteration [Li et al., 1997], and deletions of ELN are responsible for Williams–Beuren syndrome, another cardiovascular disorder [Milewicz et al., 2000]. Our data also exclude these disorders, which were initially considered in the differential diagnosis of the patients studied.
In conclusion, we report the exclusion of 14 connective tissue disorder genes in an ATS family, thus confirming that ATS is a distinct entity due to the alteration of another gene probably involved in the maintenance of a correct connective tissue organization with particular reference to intactness of the arteries.




