Mosaic supernumerary inv dup(15) chromosome with four copies of the P gene in a boy with pigmentary dysplasia
Abstract
Association of the pink-eye-dilution gene (P) with hypopigmentation is seen in patients who have oculocutaneous albinism type 2 (OCA2) and Prader–Willi syndrome (PWS) or Angelman syndrome (AS). However, it remains unknown whether duplication or amplification of the P gene causes hyperpigmentation. We previously reported a woman who had hyperpigmentation with a duplication of the proximal part of 15q, including the P gene. Here, we describe an additional patient with mosaicism of inv dup(15) and clinical manifestations of severe psychmoter retardation, epilepsy, and pigmentary dysplasia showing mottled and linear patterns of hyperpigmentation. His karyotype was 47,XY,+idic(15)(pter→q14::q14→pter)[38]/46,XY[12] de novo. Chromosomal fluorescence in situ hybridization (FISH) showed six copies of the P gene. Therefore, his cutaneous mosaicism might be caused by the presence of both normal and hyperpigmented skin due to multicopies of the P gene. © 2003 Wiley-Liss, Inc.
INTRODUCTION
About one-fourth of patients with either Prader–Willi syndrome (PWS) or Angelman syndromes (AS) have chromosomal deletions of 15q11-q13 and exhibit hypopigmentation of the skin, hair, and eyes [Butler, 1989; King et al., 1994]. Patients with oculocutaneous albinism type 2 (OCA2) due to homozygous loss-of-function mutations of the pink-eye-dilution gene (P) located at 15q11.2-q12 also manifest generalized hypopigmentation, frequently severe [Rinchik et al., 1993; Lee et al., 1994]. We recently reported a woman with duplication of 15q11.2-q14, including the P gene, who had generalized skin hyperpigmentation, and proposed that a duplication of the gene might account for her hyperpigmentation [Akahoshi et al., 2001].
Here, we describe another patient with linear skin pigmentation (pigmentary dysplasia) associated with mosaicism for a marker chromosome [mos inv dup(15)].
CLINICAL REPORT
The patient, a 10-year-old Japanese boy, has been followed-up for severe psychomotor retardation and epilepsy since age 4 years. His non-consanguineous parents and elder sister were all healthy and had no pigmentary dysplasia. He was born at 38 weeks of gestation with a birth weight of 3,480 g, following a normal pregnancy and delivery. Left inguinal hernia and left knee dislocation were surgically repaired in early infancy. At age 8 months, he developed head-nodding seizures (West syndrome) and was placed on ACTH. He attained head control at age 8 months, rolled over at 12 months, started to make sounds at 21 months, and walked supported at 2¾ years. His height at age 10 years was 137.6 cm (+0.4 SD), weight 36 kg (+0.7 SD), and OFC 53.5 cm (+0.3 SD). He had frontal bossing, mid-face hypoplasia, down-slanting palpebral fissures, a high-arched and narrow palate, coxa vara, muscular hypotonia, sensorineural deafness, and frequent complex seizures. Pigmentary dysplasia of the skin was present with normally pigmented areas surrounded by larger, hyperpigmented areas: ill-demarcated mottled patterns in the back, large-macular patterns in the abdomen, and linear patterns following the Blaschko's lines in the posterior thighs and legs [Ohashi et al., 1992] (Fig. 1). He could neither walk nor speak, and his developmental age was estimated at 9 months.
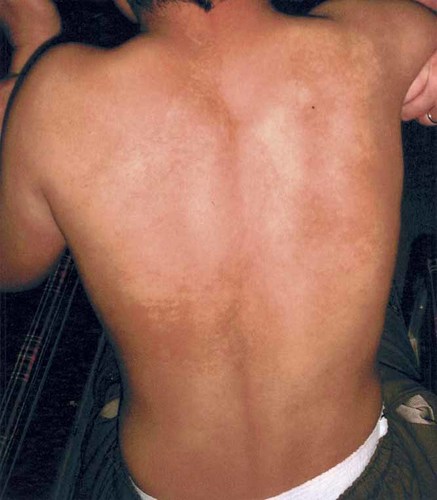
The boy with mosaic patterns of skin hyperpigmentation.
G-banded chromosome analysis of cultured peripheral blood lymphocytes from the boy showed mosaicism composed of cells with a supernumerary bisatellited marker chromosome and those with a normal karyotype (Fig. 2). Fluorescence in situ hybridization (FISH) was carried out using Vysis Prader–Willi/Angelman region probes that contains D15Z1 at the 15cen region (spectrum green), SNRPN at the 15q11-q13 PWS/AS critical region (spectrum orange), and PML at 15q22 (spectrum orange). The marker chromosome was double positive for D15Z1 and quadruple positive for SNRPN (Fig. 3A). One of chromosomes 14 was D15Z1 positive in both the abnormal and normal cell lines, and thus was interpreted a normal variant (Fig. 3A). FISH analysis using as a probe a clone spanning exons 12–14 of the P gene, distal to SNRPN [Lee et al., 1995] showed quadruple signals on the maker chromosome and a single signal on normal chromosomes 15 (Fig. 3B). His karyotype was thus interpreted as 47,XY,+idic(15)(pter→q14::q14→pter)[38]/46,XY[12].ish idic(15)(D15Z1++, SNRPN++++, P++++). G-banded chromosomes of his parents and sister were all normal. FISH analysis showed the presence of a variant chromosome 14 in both the mother and sister but not in the father. None of them had multicopies of SNRPN.
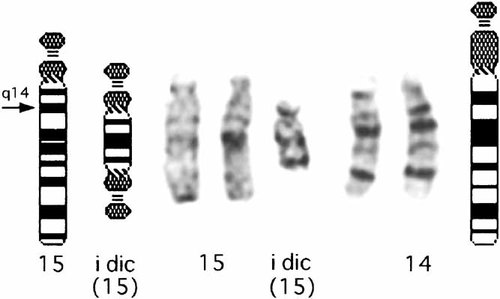
G-banded partial karyotype of the boy, showing an additional inv dup(15) chromosome.
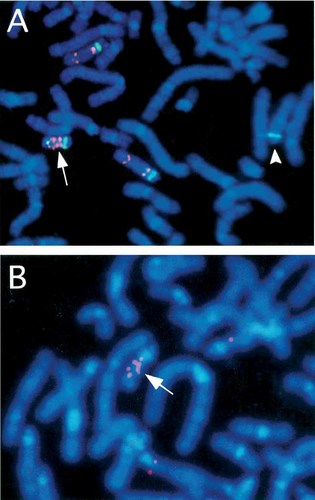
Fluorescence in situ hybridization (FISH) analysis using Vysis PWS/AS region probe (A), and partial P gene probe (B). The inv dup(15) chromosome (arrow) is double positive for D15Z1 (green), quadruple positive for SNRPN (pink), and quadruple positive for the P gene (arrow). Probe D15Z1 cross-reacts with a normal chromosome 14 (arrowhead).
DISCUSSION
The boy we described had severe psychomotor retardation and developed complex seizures, common findings in the individuals with an additional inv dup(15) chromosome containing the PWS/AS critical region [Battaglia et al., 1997]. The 15q11-q13 region is known to be unstable, probably due to the presence of region-specific low-copy repeats, and to be frequently involved in structural rearrangements such as deletion, duplication, triplication, and formation of inverted duplication. He also had mottled and linear skin patterns of hyperpigmentation. The skin patterns may reflect different copy numbers of the P gene in his two cell lines, i.e., six copies in cells with an extra inv dup(15) chromosome versus two copies in those with a normal karyotype. This hypothesis is potentially supported by our previous finding in a woman with generalized hyperpigmentation associated with a 15q11.2-q14 duplication, including a duplication of the P gene [Akahoshi et al., 2001]. PWS or AS patients hemizygous for the P gene are usually hypopigmented, regardless of the composition of their intact P allele [Spritz et al., 1997]. There was a boy with a mosaic 15pter-q13 deletion and hypomelanosis of Ito [Pellegrino et al., 1995]. All these findings suggest that changes of the P gene copy number are related to abnormal skin pigmentation: its decreased copy number is associated with hypopigmentation, while increased number leads to hyperpigmentation. Further studies in individuals with a mosaic or non-mosaic supernumerary inv dup(15) chromosome containing the PWS/AS critical region may shed light on the correlation between the P gene copy number and skin pigmentation. However, the fact that the P gene is not imprinted, and its heterozygous mutations never result in detectable hypopigmentation seems to be in apparent contradiction with our gene-dosage hypothesis. Skin pigmentation is a quantitative trait determined by both polygenes and environmental factors. Obviously, there is a room left to learn about the function and metabolism of the melanocyte.
Acknowledgements
We thank Professor Tadashi Kajii, Professor Toshiro Nagai, and Professor Takeo Kubota for their helpful suggestions and comments.




