Maternal inheritance in cyclic vomiting syndrome with neuromuscular disease
Abstract
Cyclic vomiting syndrome (CVS), characterized by severe discrete episodes of nausea, vomiting, and lethargy, is a predominately childhood condition associated with migraine and dysautonomic features. Disease-associated mitochondrial DNA (mtDNA) sequence variants are suggested by a strong maternal bias in the inheritance of migraine, and the recent findings of mtDNA variants in a few children with CVS and additional neuromuscular disease manifestations (“CVS+”). A clinical interview using a questionnaire was administered (generally) to one parent of 62 children with CVS+. Non-senile disease manifestations, including migraine, myopathy, seizures, and dysautonomia-like symptoms, were far more common in matrilineal versus non-matrilineal relatives, including being present in 75% of the mothers versus in only 11% of the fathers (P < 0.001). Overall, maternal inheritance is suggested in 86% of the families (in 65% strongly so). Disease manifestations in subjects and their affected matrilineal relatives are predominately intermittent and consistent with dysautonomia, including increased vital sign fluctuations. Body fluid metabolites and muscle biopsy findings are consistent with mitochondrial dysfunction in most cases tested. We conclude that mtDNA sequence variants are at least risk factors in the development of disease in most children at this “severe” end of the CVS spectrum, likely involving a maternally inherited propensity towards dysautonomia. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Cyclic vomiting “syndrome” (CVS) is a disabling condition characterized by recurrent, distinct episodes of nausea, vomiting, and lethargy separated by asymptomatic intervals [Li, 1995; Fleisher, 1995]. Although the duration and other characteristics of episodes vary, most patients encounter recurrent identical episodes. Episodes usually are severe and can result in frequent hospitalizations for dehydration. Episodes can be cyclical with a constant interval and/or be triggered by psychological or physiological stress [Li, 1995; Fleisher, 1995]. Cases most commonly present in the preschool years, although the disorder can start at any time from infancy to adulthood. Although the prevalence is not clear, CVS is not rarely encountered in some pediatric gastroenterology practices [Abu-Arafeh and Russell, 1995; Li, 1995].
At least 2/3 of CVS cases meet criteria for abdominal migraine1 if episodes of nausea and vomiting are substituted for those of abdominal pain and headache. In at least most cases, CVS is widely thought to be a (predominantly) childhood manifestation of a migraine-like condition [Lanzi et al., 1983; Jernigan and Ware, 1991; Forbes, 1995; Good, 1995; Symon and Russell, 1995; Li et al., 1998, 1999]. At least a third of cases evolve to migraine headaches by adulthood [Li et al., 1999]. Among children with CVS, a family history of migraine headaches is present in 80% of families [Li et al., 1999] and in 56% of first-degree relatives [Symon and Russell, 1995]. Abnormal autonomic regulation has also been documented in CVS as manifested by an increased low frequency/high frequency ratio on power spectral analysis of beat-to-beat heart rate variability [To et al., 1999] and low postural adjustment ratio of upper extremity capillary flow as measured by infrared photoplethysmography [Rashed et al., 1999]. Despite these observations, the etiology and pathophysiology of CVS are essentially unknown.
In 170 children with CVS and a family history of migraine, migraine was identified on the maternal side only in 63%, versus the paternal side only in 16% [Li et al., 1999]. As mitochondrial DNA (mtDNA) generally is derived solely from the ova and is thus “maternally inherited” [Shoffner and Wallace, 1995], this observed bias could indicate that mtDNA sequence variants are causal and/or risk factors for the development of CVS. In fact, cyclic vomiting has been reported in five children with various mtDNA mutations [Boles et al., 1997, 1998; Ito et al., 2001]. Several additional cases were highly suspected of harboring cryptic mtDNA sequence variants due to the presence of biochemical, histiological, and/or enzymatic markers of deficient energy metabolism and pedigrees demonstrating strong maternal inheritance of variable manifestations [Boles and Williams, 1999]. As often reported in families segregating known mtDNA mutations, especially the relatively common A3243G mutation [Ciafaloni et al., 1992; Harrison et al., 1997], these families show substantial intrafamilial clinical variability in terms of symptoms, age of onset, and/or severity. Common manifestations noted among the matrilineal relatives of CVS cases with confirmed or highly suspected mtDNA mutations included migraine headache, cyclic vomiting, muscle pain, depression, gastrointestinal (GI) dysmotility, and exercise intolerance [Boles and Williams, 1999].
Although most children with CVS are otherwise healthy and of normal intelligence, a significant subset have co-existing neuromuscular disorders, including cognitive delay, myopathy, and/or seizure disorders [Fleisher, 1995; authors' unpublished observations]. In this subset, neuromuscular and possibly other, disease manifestations exist between episodes, yet nausea, vomiting, and lethargy are confined to episodes. The proportion of CVS cases with this severe phenotype, here termed “CVS+,” varies depending on ascertainment, with higher proportions seen by neurologists and geneticists than by gastroenterologists (authors' unpublished observations). Among the 268 CVS cases listed on the “International CVSA Research Registry” maintained by the Cyclic Vomiting Syndrome Association USA/Canada (CVSA), 24% meet our definition for CVS+.
Cognitive delay, myopathy, and/or seizures are commonly encountered in children with mtDNA mutations [Chinnery and Turnbull, 1997], including each of the above referenced cases with CVS and confirmed or highly suspected mutations. Thus, we hypothesized that mtDNA mutations and/or associated sequence variants may be more pronounced in the CVS+ subgroup than among CVS cases in general. In order to further determine the relationship between mitochondrial dysfunction and CVS+, clinical interviews were conducted regarding 62 unrelated affected individuals, including family history, episode parameters, treatment history, associated non-CVS disease manifestations, and existing biochemical testing.
METHODS
Subjects
Forty-five of the 62 subjects were recruited through efforts of the CVSA, including the organizational web-site (now at www.cvsaonline.org) and Fall 1998 CVSA newsletter (Code “V”). The CVSA is a parent-led organization dedicated towards family support, education, and research on CVS. The remaining 17 subjects were recruited among the patients of the first author.
All subjects met the research definition of CVS [Fleisher, 1995; Li, 1995], including the presence of recurrent, severe, discrete episodes of vomiting, various intervals of (baseline) health between episodes, duration of vomiting episodes ranging from hours to days, and the absence of a specific diagnosis thought to be causal [Li et al., 1998]. Potential subjects were also excluded for the presence of a confirmed diagnosis known to result in multi-system developmental defects, and thus possibly to be a cause for CVS. Specifically, subjects were excluded for malrotation, intracerebral tumor, fetal alcohol syndrome, an abnormal karyotype (Down and Williams syndromes), or a known metabolic disorder (ornithine transcarbamylase deficiency and maple syrup urine disease). Subjects were not excluded for migraine, a suspicion of a metabolic disorder, or non-specific findings thought to be secondary to CVS such as esophagitis or gastritis. Obviously, we did not exclude the few cases in which mitochondrial disease was suspected (but never confirmed) prior to entrance into this study.
In addition to CVS, the entrance criteria were designed to recruit those individuals with CVS thought to be at higher risk for harboring mtDNA sequence variations related to their disease. All individuals on the CVSA database or patients of the first author meeting at least two of the five criteria listed in Table I, either past or present, (defined here as “CVS+”) were asked to participate as subjects.
| All subjects demonstrated two or more of the following |
| Global cognitive delay: I.Q. or D.Q.< 70 |
| Seizure disorder: any kind except simple febrile seizures |
| Myopathy: skeletal (hypotonia, weakness), ocular (usually strabismus), or cardiac; or peripheral neuropathy |
| Growth retardation: weight, height, or head circumference below the fifth percentile |
| Family history suspicious for maternal inheritance: migraine, CVS, idiopathic multi-system disease, or any of the above listed neuromuscular conditions (1–3) in either the mother, or in two of the mother's first-degree matrilineal relatives (defined in text) |
A questionnaire was administered to one parent of each minor subject, or directly to each of the few adult subjects. The questionnaire inquired about demographics, parameters of symptomatic episodes, treatment history, study entrance criteria, potential co-existing medical problems, family history (maternal and paternal equally), and available laboratory results.
Regarding each subject's and their relatives' past and present medical problems, the questionnaire focused mostly on those conditions commonly reported among individuals with mtDNA mutations: neuromuscular and visceral organ-system dysfunction, especially that of a transient nature. Only “non-senile” clinical manifestations were tabulated; i.e., common age-related problems such as coronary artery disease, cancer, hypertension, and diabetes were not considered in individuals with onset over age 30 years. Headaches were recorded as “migraine” only if chronic, severe, and either: (1) the interviewee was specifically told that diagnosis in the past, or (2) headaches are associated with significant nausea, aura, and/or sensory disturbances. Developmental delay was recorded in children with tested IQ < 70, or in children not tested, if both motor and language function based upon the interview were clearly less than a developmental level of 70. Answers were reviewed by the first author, and supporting documentation was requested when in doubt, especially regarding growth charts and past intellectual assessment data.
All qualifying subjects that the investigators contacted consented. This study was approved by the Institutional Review Board.
Data Analyses
Since mtDNA is essentially inherited only from the ova, individuals in any “matrilineage,” defined as those individuals related through all females, should share the same mtDNA sequence in the absence of a recent mutation [Shoffner and Wallace, 1995]. For the purpose of this study, analysis of pedigrees was confined to both parents of the subject and the parents' first-degree relatives. Thus, “matrilineal” relatives in this study include the subject's mother, full siblings, and maternal half-siblings, aunts, uncles, and grandmother. “Non-matrilineal” relatives in this study include the father and paternal half-siblings, aunts, uncles, and grandparents, as well as the maternal grandfather. Most subjects have 0–2 siblings, thus having near equal numbers of maternal and non-maternal relatives. Inheritance patterns were evaluated while blinded to the remaining data.
Unless otherwise stated, figures are listed as mean ± standard deviation. Statistics were performed by two-tailed Students t-test, Fisher exact test, or Yates-corrected Chi-square, as appropriate.
RESULTS
All but four of the subjects were children (94%), with a median age of 8 years. Females outnumbered males by 38 to 21 (64%). The racial/ethnic composition was 50 Non-Hispanic-Caucasian, 6 Hispanic, 1 African-American, 1 East Asian, 1 Native-American, and 3 unknown.
Inheritance Patterns
Review of the family history data shows that non-senile clinical manifestations are far more common among matrilineal than among non-matrilineal relatives, and are especially common among the subjects' mothers. The most common single finding in family members is migraine headache (as defined in Methods), which is present in 25 (44% of the 57 cases with adequate family data) of the mothers and 5 (9%) of the fathers (P < 0.001). Several disease manifestations were noted in at least three of the mothers, yet present in zero or one of the fathers, and are listed in Table II. As depression and hypothyroidism present potential difficulties related to their commonness and higher incidence in women than in men [Kessler et al., 1993; Elliott, 2000], these two conditions were excluded from further data analyses. The remaining list of “matrilineage-associated disease manifestations” was found to be highly associated with the matrilineal as opposed to non-matrilineal relatives, including mothers versus fathers (eight fold higher incidence, P < 0.001) (Table II), and maternal grandmothers, a matrilineal relative, versus each of the other three (non-matrilineal) grandparents (10–15 fold higher incidences, P < 0.001) (Table III).
| Number of mothers | Number of fathers | |
|---|---|---|
| Migraine headaches | 25 | 5 |
| Hypothyroidism | 9 | 0 |
| Muscle weakness and/or severe exercise intolerance | 7 | 0 |
| Depression requiring medications | 6 | 1 |
| Cyclic vomiting | 5 | 0 |
| Seizure disorders (excluding simple febrile) | 4 | 0 |
| Neurovascular dystrophy-like manifestations | 4 | 0 |
| Episodic “hypoglycemia” | 4 | 0 |
| Peripheral neuropathy | 3 | 0 |
| One or more of the abovea | 43 (75%b) | 5 (9%b)* |
| Two or more of the abovea | 23 (40%b) | 0* |
- *P < 0.001.
- a Excluding hypothyroidism and depression
- b Of 57 cases with adequate data
| Number affected | |
|---|---|
| Maternal grandmothers | 30* |
| Paternal grandmothers | 2 |
| Maternal grandfathers | 3 |
| Paternal grandfathers | 2 |
- Excluding hypothyroidism and depression. *P < 0.001 compared to each of the other grandparents
We defined cases to have “probable maternal inheritance” if the mother and at least one of her (first-degree) matrilineal relatives, but not the father or any non-matrilineal relatives, have one of these matrilineage-associated disease manifestations. As migraine is common in the general population (12%) [Lipton et al., 2001], an exception to the requirement of total absence of non-matrilineal involvement was made in cases where one adult non-matrilineal relative suffers from migraine. By these criteria, “probable maternal inheritance” was assigned in 37 of the 57 cases with adequate data (65%). An additional 12 cases appeared to demonstrate maternal inheritance by gestalt, but did not fulfill our criteria due to a single exception (i.e., lack of involvement in the mother or another matrilineal relative, or involvement of one non-matrilineal relative (other than migraine)). These cases were assigned as “possible maternal inheritance.” Together, 48 of 57 families (86%) demonstrated probable or possible maternal inheritance. Pedigrees in four families that were ascertained by the first author and assigned as “probable” or “possible” maternal inheritance are drawn in Figure 1.
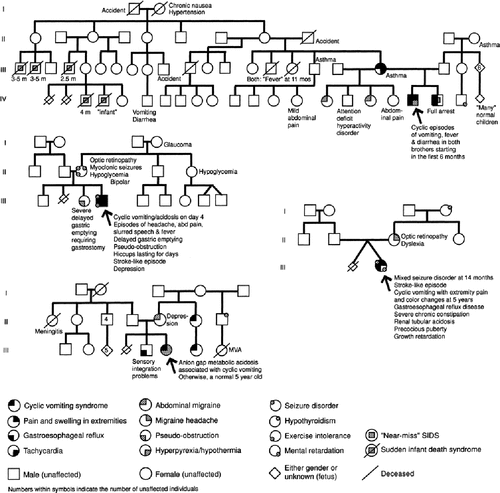
Pedigree in four families suggestive of maternal inheritance: three “probable” and one “possible” (lower right).
Family histories were also evaluated for potential Mendelian inheritance patterns. Regarding potential autosomal recessive inheritance, 24 cases had at least one sibling affected with one or more of the above listed disease manifestations. However, in 21 of these 24 families the mother was also affected, and each of the remaining three families had at least one non-sibling affected matrilineal relative. Consanguinity was present in only one family, a sporadic case with first cousin parents. Autosomal dominant and X-linked inheritance cannot be ruled out in many of the families listed as probable or possible maternal inheritance. However, in no case was father to child transmission present, and brothers and sisters of subjects were affected in near equal numbers (16 vs. 13) and severity.
CVS Parameters and Treatment in our CVS+ Subjects
The age of onset of cyclic episodes of vomiting was quite variable with a median of 3 years (3.9 ± 6.5 years, range: birth to 42 years.). The median age was also 3 years in both the probable maternal inheritance and negative family history subgroups, although the variation was greater in the former, including a trend towards more cases reporting the onset of cyclic episodes in the first year of life (11/37 vs. 1/10). One half (31) of all cases reported suffering from chronic vomiting, usually attributed to gastroesophageal reflux, months or years prior to the onset of characteristic cyclic episodes. The mean interval between (the start of sequential) episodes was 1.5 ± 1.4 months. The duration of episodes varied substantially among subjects, with a mean of 3.6 ± 4.0 days, including 4.0 ± 4.7 days among cases with probable maternal inheritance versus 2.2 ± 1.5 days in cases with a negative family history.
Prodromic symptoms were reported as being present prior to the onset of nausea and vomiting in 2/3 of the cases overall, while the episodes themselves were defined by the presence of vomiting. The prodromic and intra-ictal symptoms recorded are representative of CVS in general and are tabulated in Table IV. Therapeutic approaches have varied tremendously among physicians treating the study population. Just over half of the subjects (33) were tried on a prophylactic “anti-migraine” medication, the most commonly used were the tricyclic “antidepressants,” especially amitriptyline, with a positive response rates of 17/22 (77%) overall. In three children, amitriptyline was discontinued because of attributed side effects of a prolonged QTc, behavioral changes, or frequent urinary tract infections, all reversible after discontinuation. Cyproheptadine and propranolol were beneficial in 8/14 (57%) and 6/13 (46%), respectively. Episodes responded favorably to carbohydrate, administered either intravenously or enterally, in 35/60 subjects (58%), while the remainder reported no benefit or did not know. There was a possible trend towards greater response to carbohydrate in cases with probable maternal inheritance: 21/38 (55%) versus 3/9 (33%) in sporadic cases. Almost half (24/52) of subjects reported improvement with intravenous ondansetron given during episodes. Other therapies listed by the families to be consistently effective in at least two subjects included sedation (eight, especially lorazepam), promethazine (seven), sumatriptan, anticonvulsants, caffeine, and dexamethasone.
| Number of cases | Percenta (%) | |
|---|---|---|
| Prodromic symptoms | ||
| Altered mentation: lethargy, irritability | 23 | 42 |
| Altered secretions: tearing, drooling | 12 | 22 |
| Pallor | 4 | 7 |
| Headache | 4 | 7 |
| Any prodromic sign/symptom | 39 | 68 |
| Intra-ictal symptoms | ||
| Vomiting | 57 | 100 (by definition) |
| Lethargy and/or irritability | 50 | 91 |
| Nausea | 46 | 81b |
| Photophobia | 34 | 62 |
| Abdominal pain | 33 | 60b |
| Headache | 32 | 58b |
| Diarrhea | 11 | 20 |
| Altered secretion | 10 | 18 |
| Pallor | 7 | 13 |
| Any intra-ictal sign/symptom other than vomiting | 57 | 100 |
- a Among the 57 cases with adequate data.
- b Likely under-recognized in mentally retarded cases.
Except as stated above, no significant differences or apparent trends were noted among the various apparent inheritance pattern sub-groups.
Non-CVS Clinical Manifestations in our CVS+ Subjects
Neuromuscular/cognitive disease manifestations are common in our CVS+ population (92% in aggregate) and are tabulated in Table V. Although, the recorded incidences are somewhat biased since study recruitment was based to some degree upon their presence, these figures are given in order to document our study population. Another biased parameter, growth retardation, was also common (59%), with a trend toward a higher incidence among those with a negative family history (7/9 = 78% vs. 16/37 = 43% with probable maternal inheritance, 0.05 < P < 0.10). Among conditions that are not biased due to our study entrance criteria, GI dysmotility (71%) and other manifestations consistent with dysautonomia (65%) are very common in our study population, while exercise intolerance (45%), endocrinopathies (24%), and psychiatric disorders (24%) are common (Table V).
| Number of cases | Percent (%) | Comments | |
|---|---|---|---|
| Neuromuscular disorders (except mental retardation) | 53a | 85a | |
| Skeletal myopathy: hypotonia or weakness | 37a | 60a | |
| Seizure disorder: all types excluding simple febrile | 28a | 45a | |
| Strabismus | 23a | 37a | |
| Ataxia | 14a | 23a | |
| Cranial nerve palsies | 11a | 18a | |
| Strokes or “stroke-like” episodes | 7a | 11a | 6/7 in children |
| Cardiomyopathy | 6a | 10a | |
| Cognitive disorders | 48a | 77a | |
| Mental retardation: IQ or DQ < 70 | 34a | 55a | |
| Learning disabilities | 7 | 11 | In non-retarded cases |
| Autism, Asperger syndrome or pervasive developmental disorder | 6 | 10 | |
| Attention deficit hyperactivity disorder | 4 | 6 | In non-retarded cases |
| Gastrointestinal dysmotility | 44 | 71 | |
| Gastroesophageal reflux disease | 36 | 58 | |
| Delayed gastric emptying | 16 | 26 | |
| Constipation | 13 | 21 | |
| Irritable bowel disease/alternating constipation & diarrhea | 8 | 13 | Pseudoobstruction in 4 |
| Dysautonomic-like findings (except dysmotility) | 40b | 65b | |
| Migraine headaches | 25 | 40 | |
| Vital sign fluctuations | 19b | 31b | |
| Neurovascular dystrophy-like findings | 17b | 27b | |
| Growth retardation | 37a | 59a | |
| Stature < 5th centile | 22a | 35a | |
| Weight<5th centile | 19a | 31a | |
| Head circumference < 5th centile | 19a | 31a | |
| Exercise intolerance (substantially less than peers) | 28 | 45 | |
| Birth defects | 20b | 32b | |
| Ventricular septal defect | 4b | 6b | |
| Agenesis of the corpus callosum | 3b | 5b | |
| Spina bifida | 2b | 3b | |
| Hydroureters | 2b | 3b | |
| Endocrinopathies | 15 | 24 | |
| Thyroid disease (mostly hypothyroidism) | 8 | 13 | |
| “Hypoglycemia” | 2 | 3 | |
| Syndrome of inappropriate antidiuretic hormone | 2 | 3 | |
| Growth hormone deficiency | 2 | 3 | |
| Precocious puberty | 2 | 3 | |
| Psychiatric disorders | 15 | 24 | |
| Depression/bipolar | 6 | 9 | |
| Obsessive-compulsive disorder | 3 | 5 | |
| Tourette syndrome | 2 | 3 |
- a Likely overestimated as one of several study entrance criteria.
- b Likely underestimated as question(s) not asked of all subjects.
After verbally administering the questionnaire, parents/subjects were asked to list any additional disease manifestations present. These “write-in” anomalies were brought to our attention after the first about 30 families were interviewed, and thus the incidences listed in Table V are likely to be underestimates. Neurovascular dystrophy (NVD)-like findings were recorded in the one quarter of subjects reporting soft tissue pain, swelling, or changes in skin color or temperature, mostly in the distal extremities and often in a glove or stocking-like distribution. Most cases with NVD-like findings were found among children in the probable maternal inheritance subgroup (15/17, P = 0.10). Only 4 of these 17 reported a co-existing psychiatric condition2 (18 vs. 24% of the entire subject group). Erratic and frequent vital sign fluctuations consistent with dysautonomia are common in our CVS+ subjects (31%) and included high and/or low heart rate, respiratory rate, and/or body temperature. Most unexpectedly, significant birth defects requiring medical and/or surgical therapy were found in a third of the subjects.
Again, except as stated above, no substantial or significant differences were noted among the various apparent inheritance pattern sub-groups.
Laboratory Analyses for Mitochondrial Dysfunction in our CVS+ Subjects
Metabolite testing in plasma and urine was highly recommended, both orally and in writing, for all subjects at the time of study recruitment. However, results are available to us in only 27 cases, one half of which were patients of the first author. Venous lactate (generally obtained fasting) was elevated at least once in 20/27 cases (74%), although most determinations demonstrated normal levels in most subjects. Urine organic acids demonstrated elevations consistent with mitochondrial dysfunction (Table VI) in 24/27 cases (89%). It is important to note that the subjects followed by the first author demonstrate great temporal variability in terms of organic acid excretion, and usually demonstrated abnormal patterns only while stressed (i.e., early in episodes and during intercurrent illnesses). In most cases, we do not know the clinical state of the other subjects when tested. Although bias regarding which cases were worked-up by the subjects' physicians cannot be ruled out (i.e., towards cases with more severe disease or familial involvement), this was not grossly apparent from the data. Muscle biopsy was ordered by the subject's physician prior to this study in 13 cases. In general, cases with more severe neuromuscular disease were biopsied, and the indication was to evaluate for a possible mitochondrial disorder. Abnormal, yet predominately nonspecific, findings consistent with a mitochondrial disorder were found in 11 of these 13 cases (Table VII).
| Metabolite/pattern | Number of cases | Percenta (%) |
|---|---|---|
| Ketones | 16 | 59 |
| Lactate | 13 | 48 |
| Fumarate and/or malate | 10 | 37 |
| Generalized organic aciduria | 7 | 26 |
| Ethylmalonate | 6 | 22 |
| Glutarate | 5 | 19 |
| Free fatty acids | 5 | 19 |
| Dicarboxylic acids | 5 | 19 |
| Any of the above | 24 | 89 |
- By a standard gas chromatography/mass spectroscopy method.
- a With values > 97.5% for age among the 27 cases in which data is available (see text).
| Finding | Number of cases | Percenta (%) |
|---|---|---|
| Elevated citrate synthase | 6 | 46 |
| Partial deficiencies of complex 1 | 4 | 31 |
| Elevations in multiple respiratory chain complexes | 3 | 23 |
| Elevated lipid content | 3 | 23 |
| Any of the above | 11 | 85 |
- a Among 13 subjects biopsied.
DISCUSSION
The present study focuses on the “severe-end” of the phenotypic spectrum among children suffering from cyclic vomiting, predominately those with additional neuromuscular disease manifestations, here labeled as “CVS+.” Our subject population is obviously biased towards extra-CVS manifestations as recruitment and enrollment was contingent on their presence. However, it is apparent that additional disease manifestations cluster together among this “severe” sub-population of CVS patients. This is witnessed by the observation that about 2/3 of our subjects (40/62) exceeded the entrance requirement of 2 out of 5 criteria, with the mode being 4 criteria. Disease manifestations which cluster in this population that were not part of the entrance criteria include GI dysmotility (71%), severe exercise intolerance (45%), migraine headache (40%), vital sign fluctuations (at least 30%), NVD-like changes of the extremities (at least 27%), endocrine dysfunction (25%), and psychiatric disease (25%).
As previously reported among CVS cases in general [Li et al., 1999], we found a strong bias towards maternal versus paternal inheritance of disease manifestations in our CVS+ study population. Whereas that previous study focused on the inheritance of migraine headache, we show here a substantial maternal bias not only for migraine, but also for several neuromuscular and other disease manifestations. Our findings that highly variable disease manifestations are very common in the mothers, common (and equally so) among the brothers and sisters, less common in second-degree matrilineal relatives, and essentially absent in the fathers and all other non-matrilineal relatives is not consistent with any form of Mendelian inheritance or multifactorial/polygenic inheritance. However, these findings are consistent with the maternal inheritance of mtDNA sequence variants. Since the family history is suggestive of maternal inheritance in 86% of our cases (65% strongly so), our data suggest that mtDNA sequence variants are at least risk factors in the majority of CVS+ cases. Although non-specific by themselves, the elevated body fluid metabolites seen in most cases tested suggest impaired carbohydrate and fat metabolism. These findings are consistent with mitochondrial dysfunction, and thus with the maternal inheritance of mtDNA variants adversely affecting energy metabolism.
Our finding of maternal inheritance among most CVS+ cases is not an artifact of assigning the existence of affected maternal relatives as a study entrance criteria, as only three cases would not have met study criteria if the family history were excluded. A potential cofounder is that some disease manifestations are more often diagnosed in women than men, including migraine [Lipton et al., 2001], depression [Kessler et al., 1993], and hypothyroidism [Elliott, 2000]. Thus, the latter two were excluded from all statistical analyses, although hypothyroidism was seen in 16% of our mothers versus a reported prevalence of 1.4–2.0% of women [Elliott, 2000]. However, as migraine is the primary manifestation recorded among matrilineal relatives of our CVS+ cases, and it itself is in some way closely related to CVS, its presence was not excluded from analyses. While the prevalence of migraine among our fathers slightly exceeds the reported population prevalence (9 vs. 6.5%, Lipton et al., 2001), the prevalence in our mothers greatly exceeds that reported (44 vs. 18.2%). If we exclude migraine, depression, and hypothyroidism from analysis, 30 mothers (53%) and 0 fathers (P < 0.001) were reported as affected.
Another potential bias and limitation is the obtaining of medical data in most cases over the telephone from a parent, usually the mother. It is conceivable that ascertainment would be more complete for the mother's own relatives than that of the child's father's relatives. However, the vast majority of the mothers expressed comfort in detailing the paternal family history and the data obtained grossly appears to be complete, including the reported prevalence of migraine among the fathers. In addition, such a reporting bias would not explain the vast difference in disease prevalence reported between the maternal grandmothers and maternal grandfathers, and the lack of significant difference in prevalence reported between the maternal and paternal grandfathers.
Many of the specific disease manifestations seen in our cases and their affected matrilineal relatives are suggestive of a dysautonomia. Those manifestations seen in our matrilineages that are common in the autosomal recessive condition familial dysautonomia (also known as Riley-Day), include cyclic vomiting, failure-to-thrive, gut dysmotility, changes in size, color, or warmth of the distal extremities and dysregulation of heart rate and body temperature [Slaugenhaupt et al., 2001]. In addition, several of our cases have “spells” which resemble dysautonomic crises including irritability, headache, pallor, syncope, and clonic movements with a normal EEG. Migraine headache is not usually considered to be a symptom of dysautonomia, although it is not uncommon in Riley-Day [Personal communication Sonia Peltzer, M.D., President, Familial Dysautonomia Hope, Inc.], and neurovascular dysautonomia has been proposed as a potential cause of migraine [Cortelli, 1993; Lehmann et al., 1996]. As previously stated, abnormal autonomic function has been reported in two series of children with CVS, and has been considered to be part of its cause and/or pathogenesis [Rashed et al., 1999; To et al., 1999]. In addition, dysautonomia has been described previously in children with mitochondrial disease [Sasaki et al., 1995; Zelnik et al., 1996; Haftel et al., 2000].
The most characteristic finding in our cases and their affected matrilineal relatives is the presence of intermittent and transient clinical manifestations, often temporally clustered, and triggered by physiological or psychological stress. This pattern is common in familial dysautonomia as well as in many cases of mitochondrial disease, possibly in the latter also due to the presence of dysautonomia.
In conclusion, our data demonstrate that neuromuscular and other clinical manifestations cluster in a “severe” group of CVS sufferers. Maternal inheritance is present in most cases, which along with the associated clinical and laboratory findings, strongly suggests that mtDNA sequences variants are at least risk factors in the development of most cases of CVS+. Associated mtDNA sequence variants were indeed found in several cases, including in many of the present subjects (manuscript in preparation). Many of the clinical findings seen in our cases, including cyclic vomiting itself, are also found in dysautonomia, and our data offers further support that dysautonomia is involved in the pathophysiology of CVS+/CVS. In addition, most of our subjects have matrilineal relatives, especially their mothers, who also suffer from varied disease manifestations, most of which are also consistent with dysautonomia. Thus, we propose that it is a propensity towards dysautonomia that is maternally inherited, with CVS being only one of several potential clinical manifestations. Physiological studies are in progress to confirm and characterize the presence of dysautonomia in these subjects and their family members, as well as efforts to extend our analyses to an unbiased group of CVS patients.
Our finding of a strong mitochondrial factor in the cause of CVS+ has immediate and practical application to disease management in these children. For example, anecdotal experience with our own patients has demonstrated that frequent, relatively low-fat feedings and the timely administration of intravenous 10% glucose solutions can decrease the frequency and duration, respectively, of vomiting episodes in the majority. In most cases, combination anti-metabolic/mitochondrial disease (i.e., fasting avoidance) and anti-migraine (i.e., amitriptyline) therapy is highly effective. Genetic counseling is very complicated, although it is important to note that, by present definition, more than one family member is affected, and often multiple matrilineal relatives can benefit from simple therapies such as fasting avoidance.
Acknowledgements
We generously thank the clinicians for patient referral, and all of the families, including the CVSA, without whose patience, insight, and fundraising this work would not have been possible.




