CHARGE association and secondary hypoadrenalism
Abstract
Hypogonadotrophic hypogonadism is a well-described part of the CHARGE association. It has been suggested that more extensive defects of the pituitary function may occur, but this has not been reported in the medical literature. We report a patient with CHARGE association who, in addition to the cardinal manifestations, was found to have hypoadrenalism of pituitary/hypothalamic origin. The patient also has findings typical of the overlap of CHARGE and the 22q11 deletion syndrome, as well as an unusual cervical vertebral abnormality. © 2002 Wiley-Liss, Inc.
INTRODUCTION
The CHARGE association was first described in 1981. The main manifestations include colobomas of the eyes, heart defects, choanal atresia, growth retardation, developmental delay, genital hypoplasia, and ear abnormalities or deafness [Pagon et al., 1981]. The genital hypoplasia, which is only evident in affected males, has recently been shown to be the result of hypogonadotrophic hypogonadism. This defect is present in patients of both sexes and results in failure to develop normal secondary sexual characteristics [Davenport et al., 1986; Wheeler et al., 2000].
Although there have been reports of growth hormone deficiency [August et al., 1983], in the majority of cases the growth retardation of this condition is associated with normal growth hormone responses [Oley et al., 1988]. The possibility of more extensive pituitary or hypothalamic defects has been suggested [August et al., 1983], but this has not been previously reported in the literature. We now report the case of a patient with CHARGE association who has both hypogonadotrophic hypogonadism and a defect in ACTH secretion, resulting in secondary hypoadrenalism.
CLINICAL REPORT
The patient is the first child of nonconsanguineous parents. He was born by normal vaginal delivery at 41 weeks gestation following a normal pregnancy to a 28-year-old nondiabetic mother. His birth weight was 3,125 g (10th-25th centile); birth length, 46 cm (<3rd centile); and head circumference, 35 cm (50th centile). He developed respiratory distress at delivery with Apgar scores of 2 and 5. Examination revealed microphthalmia with bilateral retinal colobomas, a grooved, tented palate, an asymmetrical crying face, a short neck, and abnormally adducted thumbs. The ears were dysplastic and low-set bilaterally (Fig. 1). Examination of the genitalia revealed micropenis and a hypoplastic scrotum with normally descended testes (Fig. 2).
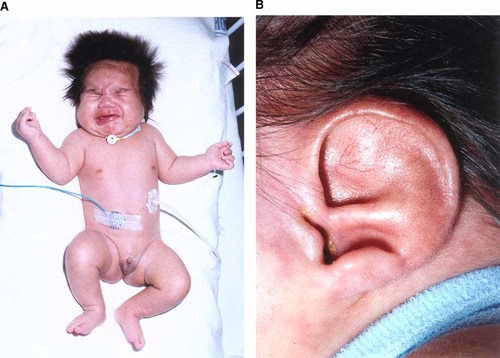
The propositus at 1 month of age. Note the asymmetric crying face (A) and simple low-set ears (B). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
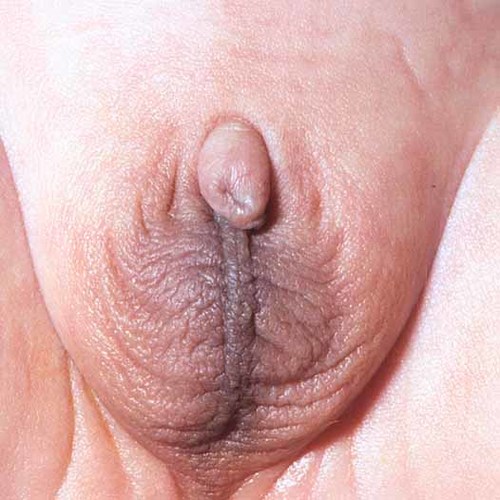
External genitalia showing micropenis and hypoplastic scrotum. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Respiratory distress continued and intubation was required. An endotracheal tube could not be passed through either nostril, but oral intubation was achieved without difficulty. Choanal atresia was later confirmed by computed tomography. A tracheostomy, performed at the end of the first week, was technically difficult due to a short neck. A bronchoscopy demonstrated supralaryngeal collapse and narrowing of the supralaryngeal introitus. There was hypoplasia and narrowing of the right main bronchus intermedius, resulting in an ongoing requirement for oxygen after ventilation had been weaned.
A roentgenograph of the cervical spine revealed basilar invagination, a hypoplastic C3 vertebra, and a constricted skull base (Fig. 3). The chest roentgenograph showed handlebar deformity of the clavicles, but a normal thymic shadow. A loud systolic murmur was heard on auscultation, and a widely patent ductus arteriosus was detected on echocardiography. No defects were seen within the heart. A karyotype performed at birth showed a normal male compliment of 46,XY.
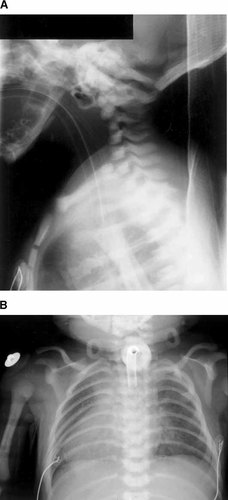
Roentogenographs. A: The lateral cervical spine showing hypoplasia of the body of the C3 vertebra, basilar invagination, and constriction of the skull base. B: AP chest showing the handlebar deformity of the clavicles.
In the first 24 hr the patient developed problems with hyponatremia, and hypoglycemia that persisted despite up to 11 mg/kg/hr of intravenous dextrose. Initial endocrine investigations suggested hypogonadotrophic hypogonadism (testosterone, 0.4 nmol/L; FSH and LH, <0.08 IU/L), which was confirmed by failure to respond to a gonadotrophin-releasing hormone (GnRH) stimulation test (laboratory results, Table I). In addition, tests revealed hypoadrenalism, with a low baseline cortisol level and an inappropriately normal ACTH of 3.4 pmol/L. There was inadequate response to low-dose intravenous (1 μg) Synacthen stimulation, with serum cortisol peaking at 360 nmol/L at 1 hr. These results indicate insufficiency at the pituitary/hypothalamic level. Growth hormone secretion and thyroid function tests were normal. He was commenced on hydrocortisone replacement, and diazoxide resulting in normalization of both the sodium and glucose levels.
| Baseline | Post-stimulation | Normal values | |
|---|---|---|---|
| Testosterone (nmol/L) | 0.4 | 11–35 | |
| FSH (IU/L) | <0.8 | <0.8a | 0.8–3.4 |
| LH (IU/L) | <0.8 | 1.1a | 0.8–1.7 |
| Cortisol (nmol/L) | 47 | 360b | 200–550 |
| ACTH (pmol/L) | 3.4 | 2.0–11.0 | |
| HGH (μg/L) | 37.3c | >7.5 | |
| T4 (pmol/L) | 20.0 | 15.7–39.2 | |
| TSH (mIU/L) | 7.0d | 0.4–4.0 | |
| Calcium (mmol/L) | 1.74 | 2.12–2.64 | |
| Phosphate (mmol/L) | 1.66 | 1.25–2.50 | |
| PTH (pmol/L) | 1.0 | 1.0–5.0 | |
| 25-OH Vitamin D (μg/L) | 9.0 | 14–76 |
- * Adjusted calcium, phosphate and PTH results are from samples on the same day.
- a FSH and LH stimulation = IV GnRH 250 μg, normal values FSH 1.5–10.8, LH 1.5–11.9.
- b Normal response = a rise in cortisol of at least 200 nmol/L to >550 nmol/L at one hour.
- c Taken during hypoglycaemia.
- d TFTs taken in second week of life, TSH remains elevated from initial surge following delivery.
- FSH, follicle stimulating hormone; LH, luetinising hormone; ACTH, adrenocorticotrophic hormone; HGH, human growth hormone; T4, thyroxine; TSH, thyroid stimulating hormone; PTH, parathyroid hormone.
Audiological assessment at 1 month of age showed no brain stem responses to high- or low-frequency tones, as well as to bone conduction, suggesting bilateral severe or profound sensorineural hearing loss.
At the end of the first week he was noted to be hypocalcemic, with low 25 hydroxy-vitamin D and an inappropriately normal parathyroid hormone. These findings were consistent with hypoparathyroidism and concomitant vitamin D deficiency. Calcium gluconate and 1 hydroxy-vitamin D replacement were required. Analysis of T-cell populations demonstrated low numbers of mature T-cells, consistent with the DiGeorge anomaly. Examination of the DiGeorge region of chromosome 22 by fluorescence in situ hybridization (FISH) with the TUPLE1 probe found no evidence of a microdeletion.
His early course was complicated by episodes of sepsis from both the bowel and respiratory tract. He was discharged home at 10 weeks of age and remains on steroid replacement without further episodes of hypoglycemia or hyponatremia. Calcium and vitamin D supplementation was stopped after 8 weeks of therapy, but hypocalcemia recurred. A persistently elevated serum phosphate indicated that there was ongoing hypoparathyroidism, and he was restarted on oral calcium and vitamin D replacement.
DISCUSSION
Our patient represents a severe case of the CHARGE association. He has malformations in each of the four major diagnostic categories, namely, retinal colobomas, choanal atresia, typical abnormalities of the ear, and cranial nerve dysfunction. In addition, he has several of the minor characteristics (hypogonadism, growth deficiency, cardiovascular anomaly) and occasional findings (the T-cell dysfunction of the DiGeorge anomaly, hand anomalies, spinal anomalies, and general appearance) that make up the diagnostic criterion [Blake et al., 1998]. These findings illustrate the previously described overlap between the CHARGE association, an asymmetric crying face, and the spectrum of abnormalities associated with deletions of 22q11 [De Lonlay-Debeney et al., 1997]. As in other cases where the findings of the DiGeorge anomaly have been found with the CHARGE association, our patient was negative when tested for the chromosomal deletion.
Testing his hypothalamic-pituitary axis confirmed that his genital hypoplasia is due to a central deficiency of gonadotrophins, as has been previously described [Wheeler et al., 2000]. An adrenal crisis on the first day following delivery involving hypoglycemia and hyponatremia drew attention to a more extensive endocrinopathy. Although previous reports have suggested that pituitary or hypothalamic deficits may be part of the spectrum of the CHARGE association [August et al., 1983], only one previous report has demonstrated abnormalities of hypothalmic/pituitary function other than hypogonadotrophic hypogonadism. August et al. [1983] reported a 17-year-old girl who in addition to a typical pattern of malformation and central hypogonadism showed subnormal growth hormone responses and an abnormally low growth velocity that was corrected by growth hormone replacement. Borderline responses to stimulation of growth hormone release have also been reported in one further case [Oley et al., 1988]. In contrast, normal growth hormone secretion has been reported in association with short stature in the majority of patients in which it has been studied, and normal growth velocity is typical.
Episodes in which babies with CHARGE association were noted to be sweaty, cold, and drowsy, and have responded to being given glucose-containing fluids, have been ascribed to hypoglycemia by their parents (personal communication), but this has not been described in the medical literature. In our patient hypoglycemia was secondary to adrenal insufficiency, as suggested clinically by the failure to improve with high-dose intravenous dextrose replacement, but rapid resolution with steroid replacement, and confirmed biochemically by random cortisol measurement and a low-dose Synacthen test. In the first 10 days of life cortisol secretion, corrected for body size, is 1.5–2 times greater than that in later childhood or adulthood, and a constant level of secretion is present until the development of diurnal variation at around 4–6 months [ Midgeon and Donohoue, 1994]. This makes the newborn very sensitive to the effects of adrenal insufficiency. Our case illustrates that this should be considered as a possible underlying etiology for an infant with CHARGE association presenting with an acute illness in the neonatal period.
The vitamin D deficiency in this infant is environmental. Maternal 25 OH vitamin D3 was also low, confirming nutritional vitamin D deficiency. This is not an uncommon problem in New Zealand in Indian and Polynesian mothers who often have a diet poor in vitamin D and who also avoid sun exposure. Correction of the vitamin D deficiency did not correct the underlying hypoparathyroidism.
Vertebral abnormalities have rarely been reported in this condition, although a short neck, suggestive of Klippel-Feil anomaly, has been described [August et al., 1983]. Underlying radiologic abnormalities of the cervical vertebrae have not been reported. Such anomalies as noted in our patient should be considered and defined radiologically as they may have significant orthopedic, anesthetic, and nursing implications.




