Molecular cytogenetic characterization of a derivative chromosome 8 with an inverted duplication of 8p21.3→p23.3 and a rearranged duplication of 8q24.13→qter
Abstract
A derivative chromosome 8 was observed in a newborn boy who presented with low birth weight, multiple congenital anomalies, and dysmorphic face. The der(8) was further characterized at age 18 months by a high resolution G-banding analysis, spectral karyotyping, and fluorescence in situ hybridization (FISH) with multiple DNA probes. The karyotype was described as 46,XY,der(8)(qter→q24.13::p21.3→p23.3::p23.3→qter), representing an inverted duplication of region 8p21.3→p23.3 and a duplication of region 8q24.13→qter, which attaches to the duplicated short arm segment at 8p21.3. Different from previously reported patients with an inverted duplication (8p), no deletion was detected in the distal region of 8p in this case. This young child had manifested a broad nasal bridge, micrognathia, cleft lip, hydrocephalus, partial agenesis of the corpus callosum, Dandy-Walker malformation, congenital heart defects, dysplastic kidneys, hydronephrosis, marked hypotonia, and significant psychomotor retardation. These features are compared with those commonly seen in cases with an inverted duplication of 8p and cases with a partial trisomy of 8q. © 2001 Wiley-Liss, Inc.
INTRODUCTION
It has been known for more than 20 years that an inverted duplication of 8p is associated with facial anomalies and severe developmental delay [Weleber et al., 1976; Taylor et al., 1977; Hongell et al., 1978; Mattei et al., 1980; Jensen et al., 1982; Fryns et al., 1985; Dill et al., 1987; Kleczkowsksa et al., 1987; Nevin et al., 1990; Gorinati et al., 1991; Feldman et al., 1993; de Die-Smulders et al., 1995; Guo et al., 1995; Floridia et al., 1996]. The inverted duplication has included the region 8p12→p23.1 in most of the cases reported. In contrast to the cases with an inverted duplication of 8p, individuals with a direct duplication of region 8p22→p23.3 have been reported to be clinically normal or have mild mental retardation with no dysmorphic features [Engelen et al., 1995; Barber et al., 1998; Engelen et al., 2000]. DNA and fluorescence in situ hybridization (FISH) studies of patients with an inverted duplication of 8p have shown a deletion in the terminal region 8p23→pter in all cases examined [Engelen et al., 1994; Guo et al., 1995; Floridia et al., 1996]. We present an 18-month-old child who had significant developmental delay and multiple congenital anomalies associated with a complex inverted duplication of regions 8p21.3→p23.3 and a duplication of region 8q24.13→qter without deletion in the subtelomeric region of 8p.
CLINICAL REPORT
This male infant was the third child born to a non-consanguineous couple after an uneventful pregnancy. His birth weight was 3.11 kg (10-25 centile), his length was 53 cm (75th centile), and his head circumference was 36.3 cm (75th centile). Apgar scores were 4 and 8 at one and five minutes. Multiple congenital anomalies were noted. He developed respiratory distress and required transfer to a tertiary care hospital.
On examination at five days of age, the baby had a weak, low cry. He was noted to have a large anterior fontanelle measuring 3 × 3 cm, bitemporal narrowing, slightly low set ears, upslanting palpable fissures, wide nasal bridge, right cleft lip, micrognathia, and excess nuchal skin. There were natal teeth with a very irregular border of the upper gingiva. His nipples were hypoplastic and widely spaced. The left testis was palpable in the inguinal canal and the right testis was in the scrotum. Two-dimensional echocardiogram revealed an atrial septal defect, membranous ventricular septal defect, and patent ductus arteriosus with a parachute mitral valve. Abdominal ultrasound revealed a right pelvic dysplastic kidney and left hydronephrosis. Computed tomography (CT) scan of the head demonstrated partial agenesis of the corpus callosum, communicating hydrocephalus, Dandy Walker malformation, and an intramedullary cord defect.
At 18 months of age (Fig. 1), he was very small and had significant delays in mental and motor skills. His weight was 7.2 kg (< 5th centile), his length was 75.8 cm (< 5th centile), and his head circumference was 49.2 cm (75th centile). His hand length was 8.6 cm (< 3rd centile) and his middle finger length was 4 cm (25th centile). The child's head was relatively large with prominent veins and his ventricular septal defect was still patent. He was markedly hypotonic, lying with hips abducted. He could roll in both directions, transfer objects from hand-to-hand, and scribble if given a pencil, but could not use a spoon or fork to feed himself. He babbled, laughed, and smiled properly but had no speech. He had been receiving speech therapy once a month and physiotherapy once a week, and an infant stimulation program every two weeks. Assessment at a chronological age of 17 months using the Bayley Scales of Infant Development (BSID-II) showed consistent cognitive skills up to six months and scattered skills to 13 months. Motor skills were solid at six months with scattered skills up to about nine months.
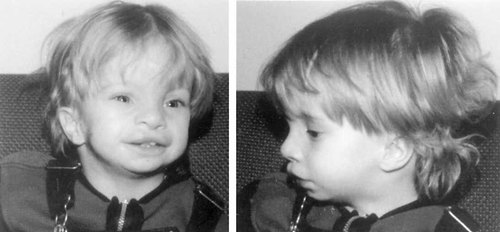
Patient at age 18 months.
MATERIALS AND METHODS
G-Banding Analysis
Chromosomes were analyzed with G-banding for the newborn at five days of age with a phytohemagglutinin-stimulated peripheral blood culture. At 18 months of age, a high resolution G-banding analysis was performed with a synchronized lymphocyte culture. The karyotype was interpreted according to the ISCN 1995.
Spectral Karyotyping (SKY)
Chromosome slides were hybridized with the 24-color SkyPaint probes according to the protocol recommended by the manufacturer of the probes (Applied Spectral Imaging, Carlsbad, CA). SKY analysis was performed with a SkyVision imaging system (Applied Spectral Imaging, Carlsbad, CA) equipped with a Zeiss Axioskop fluorescence microscope, as previously described [Fan et al., 2000]. Each individual chromosome was visualized with both display and classified images.
FISH Analysis
FISH was performed according to the protocols recommended by the manufacturers of the DNA probes. A chromosome painting probe specific to 8p (ALTechnologies, Arlington, VA) and subtelomeric probes specific to 8p and 8q (Vysis, Downers Grove, IL) were used in this study.
RESULTS
The initial G-banding analysis at five days of age revealed a large extra segment in the short arm of one chromosome 8, but the origin of the extra material could not be identified due to the limited quality of chromosome preparation and banding resolution. A karyotype of 46,XY,add(8)(p23) was reported. This was assumed to be de novo since both of the parents had a normal karyotype. At the age of 18 months, chromosomes were analyzed at the resolution level of 550-650 bands. The derivative 8 was identified to be a complex duplication involving both the short and the long arms.
Spectral karyotyping confirmed that the extra material in the der(8) had originated from chromosome 8 itself, and did not detect any interchromosomal rearrangement in this karyotype. Further FISH with an 8p specific painting probe confirmed the duplication of a short arm segment. FISH with a subtelomeric probe specific for 8q confirmed the duplication and rearrangement of a long arm distal region. FISH using a probe for the subtelomeric region of 8p showed clear hybridization signals in both the normal and the derivative chromosomes 8. Combining the results of high resolution G-banding analysis, spectral karyotyping, and FISH, the karyotype was described as: 46,XY,der(8)(qter→q24.13::p21.3→p23.3::p23.3→qter) (Figs. 2 and 3).
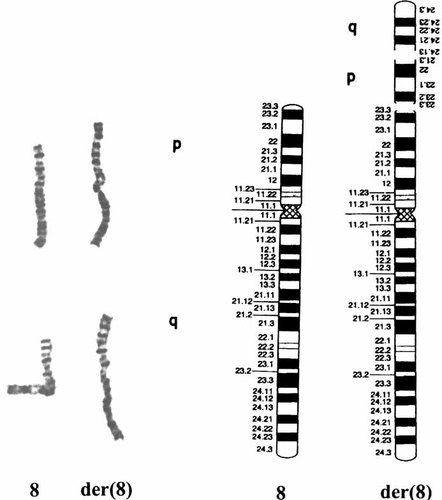
Two partial karyotypes and an ideogram showing the normal chromosome 8 and the der(8).
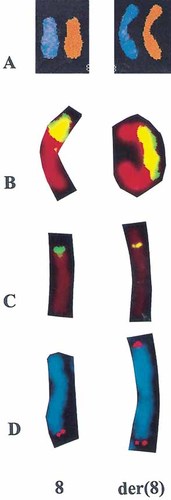
Partial karyotypes showing the normal and the der(8). A: Spectral karyotyping. B: FISH with an 8p specific painting probe. C: FISH with an 8p specific subtelomeric probe. D: FISH with an 8q specific subtelomeric probe.
DISCUSSION
The structure of the derivative 8 in our case included an inverted duplication of region 8p21.3→p23.3 and a duplication of region 8q24.13→qter that rearranged into the short arm at the duplicated band 8p21.3. Given that no deletion was detected in the subtelomeric region, this karyotype virtually represents partial trisomies of regions 8p21.3→p23.3 and 8q24.13→qter.
More than 40 cases of inverted duplication of 8p have been reported [Weleber et al., 1976; Taylor et al., 1977; Hongell et al., 1978; Mattei et al., 1980; Jensen et al., 1982; Fryns et al., 1985; Dill et al., 1987; Kleczkowsksa et al., 1987; Nevin et al., 1990; Gorinati et al., 1991; Feldman et al., 1993; de Die-Smulders et al., 1995; Guo et al., 1995; Floridia et al., 1996]. The clinical syndrome associated with an inverted duplication of 8p was recently reviewed by Feldman et al. [1993], de Die-Smulders et al. [1995], and Guo et al. [1995]. The major clinical features in young children include a history of congenital hypotonia, feeding difficulties in the neonatal period, significant developmental delay, and anomalies consisting of prominent forehead, broad nasal bridge, prominent nose with anteverted nostrils, sagging cheeks, high arched or cleft palate, micrognathia, eversion of the lower lip, large mouth, prominent ears, agenesis of corpus callosum, and skeletal anomalies such as kyphosis or scoliosis.
In contrast to inverted duplication (8p), it has been noted that a direct duplication of regions 8p22→p23.1, 8p23.1 and 8p23.1→p23.3 is not associated with obvious phenotypic anomalies [Engelen et al., 1995; Barber et al., 1998; Engelen et al., 2000; our unpublished cases].
A number of cases with a trisomy 8q23 or 24.1→qter have been reported and the clinical features were reviewed by Romain et al. [1989] and Stengel-Rutkowski et al. [1992]. The clinical manifestations of trisomy 8q region have included early feeding difficulties, developmental delay, growth retardation, mental retardation, prominent forehead, sunken temporal region, hypertelorism, broad/depressed nasal bridge, downturned/thin upper lip, micrognathia, low set and prominent ears, short fifth finger, and clinodactyly.
It has been noted that the facial traits of children with an inverted duplication (8p) strongly resemble those of patients with a mosaic trisomy 8 [de Die-Smulders et al., 1995]. From the above lists of the clinical features of inverted duplication (8p) and trisomy 8q24.1→qter, it is evident that there are many features in common. The young child described in this report had a broad nasal bridge, low set ears, bitemporal narrowing, micrognathia, cleft lip, partial agenesis of the corpus callosum, hydrocephalus, Dandy-Walker malformation, congenital heart defects, dysplastic kidneys, hydronephrosis, marked hypotonia, and significant psychomotor retardation. This child had many features that manifested in both inverted duplication (8p) and partial trisomy of 8q terminal region (Table I). While agenesis of the corpus callosum appears to be particularly common in the cases of inverted duplication (8p), hydocephalus and Dandy-Walker malformation has also been reported in one of four patients studied by brain imaging [Feldman et al., 1993].
| Characteristic | Inv dup(8p)a | Trisomy 8q24b | Present patient |
|---|---|---|---|
| Low birth weight | – | – | + |
| Feeding difficulties | + | + | ? |
| Developmental delay | + | + | + |
| Growth retardation | + | + | + |
| Mental retardation | + | + | + |
| Corpus callosum agenesis | + | – | + |
| Hypotonia | + | – | + |
| Prominent forehead | + | + | – |
| Triangular face | – | + | – |
| Hypertelorism | – | + | – |
| Eye abnormalities | + | – | – |
| Broad nasal bridge | + | + | + |
| Anteverted nostrils | + | – | – |
| Prominent nose | + | – | – |
| Bitemporal narrowing | + | + | + |
| High palate/cleft palate | + | – | – |
| Cleft lip | – | – | + |
| Downturned/thin upper lip | – | + | – |
| Eversion lower lip | + | + | – |
| Large mouth | + | – | – |
| Long flat philtrum | + | + | – |
| Low set ears | – | + | + |
| Prominent ears | + | + | – |
| Micrognathia | + | + | + |
| Short neck | + | – | – |
| Short fifth finger | – | + | – |
| Camptodactyly | – | + | – |
| Clinodactyly | – | + | – |
| Contractures of large joints | + | – | – |
| Scoliosis | + | – | – |
| Foot abnormalities | – | + | – |
| Congenital heart defects | – | – | + |
| Dysplastic kidney/hydronephrosis | – | – | + |
The breakpoints in inverted duplication (8p) have been clearly defined by high resolution G-banding and FISH analysis [Feldman et al., 1993; de Die-Smulders et al., 1995; Guo et al., 1995; Floridia et al., 1996]. The proximal breakpoint was assigned to region 8p11.2→p21 and the distal breakpoint was assigned to region 8p22→p23 or p23.1. The region 8p21→p22 was commonly duplicated in all cases. It was proposed that inverted duplication of 8p was formed due to a single U-type exchange within an inversion loop during meiosis [Feldman et al., 1993]. With this mechanism, a deletion in the distal region of 8p would be expected to occur, and it is possible that this deletion may have accounted for some of the phenotypic features of this syndrome. Indeed, a deletion in the region 8p23→pter of the derivative 8 was reported in some early cases [Dill et al., 1987; Henderson et al., 1992] and has been demonstrated in all the recent cases examined [Engelen et al., 1994; Guo et al., 1995; Floridia et al., 1996]. In our case, however, the region 8p21.3→p23.3 was duplicated without a deletion in the subtelomeric region. It is thought that the derivative chromosome 8 in our case arose as a product of meiosis recombination by a mechanism similar to that proposed by Feldman et al. [1993], but the abnormal chromosome 8 existing in germline had double inversions, i.e., a pericentric inversion of segments 8p23.3→pter and 8q24.13→qter, and a paracentric inversion of segment 8p21.3→p23.3. We assume that the breakage at 8p23.3 involved in the double inversions is within or very close to the telomere DNA sequence and a meiosis cross-over has occurred within the same sequence between the short arms of the inverted chromosome 8 and its homolog, and therefore no deletion of the subtelomeric region is expected from the recombination event.
Since this patient also had a duplication of 8q24.13→qter, we propose that the phenotypic manifestations of this case are due to a duplication of region 8p21.3→p23.3 and a duplication of region 8q24.13→qter. Given that tandem duplication of region 8p22→p23.3 is apparently not clinically significant [Engelen et al., 1995; Barber et al., 1998; Engelen et al., 2000; our unpublished cases], we further propose that the phenotypic manifestations of this case are mainly the effects of trisomy 8q24.1→qter and trisomy 8p21.3→p22, which has been commonly involved in all the cases with an inverted duplication (8p). It has been suggested by de Die-Smulders et al. [1995] that the contribution of the deletion to the clinical picture of inverted duplication of region 8p21→p22 is probably less important than the inversion duplication, because patients who have a terminal 8p deletion as the only anomaly have a very mild clinical picture [Fryns et al., 1989]. Our case may provide additional evidence to support this suggestion.
Acknowledgements
The authors thank Brenda Hamilton, Diana Munavish, and Beverley Walter for their technical assistance in cytogenetic and FISH analysis of this case.




