Clinical aspects of defects in the determination of laterality
Abstract
Of individuals in the human population, 99.99% have developed identical thoracoabdominal asymmetry with the cardiac apex, a bilobed lung, the stomach, and the spleen on the left side of the midline, and the vena cavae, a trilobed lung, the appendix, and the larger liver lobe on the right. This arrangement of organs is situs solitus. Occasionally, individuals have a complete, mirror-image reversal of this asymmetry called situs inversus, and 20–25% of those individuals have an autosomal recessive condition, Kartagener syndrome, with ciliary dyskinesia, bronchiectasis, sinusitis, and infertility. Between these extremes of situs solitus and situs inversus lies the spectrum of situs ambiguus, characterized by isomerism, heterotaxy, and multiple malformations in one or more thoracic or abdominal organs. Although most abnormal situs in humans occurs sporadically, growing evidence suggests that interference with normal genetic mechanisms and pathways may be responsible for most cases. Familial cases suggest major effects of both autosomal and X-linked genes with both dominant and recessive expression. Situs inversus and situs ambiguus (SI/SA) occurring in probands who have close relatives with “isolated,” nonsyndromic birth defects suggests that some of the pathways important in situs determination may also be involved in causing sporadic malformations not obviously associated with a defect in laterality determination. Human phenotypes of interest include the association of SI/SA with short rib–polydactyly syndromes and renal–hepatic–pancreatic dysplasia, and with agnathia and holoprosencephaly. Further elucidation of the developmental pathways involved in left–right axis determination should shed light on the causes of and relationships among these human phenotypes. © 2001 Wiley-Liss, Inc.
INTRODUCTION
The purpose of this review is to provide a brief overview of some of the patterns of malformation that occur in humans and animals that have apparent defects in the determination of laterality. It is hoped that such information will be of diagnostic help in classifying individual patients, and provide some insight about possible underlying mechanisms of normal and abnormal morphogenesis.
Most parts of the human body are symmetric about the sagittal plane. Asymmetric organs include the stomach, heart and great vessels, lungs, liver, gallbladder and biliary tract, gastrointestinal tract, and spleen. The spleen develops from the left side of the dorsal mesogastrium and is the only organ that is left sided at its inception [Van Meirop et al., 1972]. All other unpaired organs begin as midline structures and then lateralize in later development. The usual positioning of these organs is called “situs solitus” and a mirror-image reversal of this arrangement is called “situs inversus.”
SITUS SOLITUS AND SITUS INVERSUS
In situs solitus, the right lung usually has three lobes, and the right mainstem bronchus is shorter and deviates from the midline by a smaller angle than the left. The right superior lobe bronchus is called an eparterial bronchus; it arises and passes above the pulmonary artery. In contrast, the right middle and lower lobe bronchi are hyparterial. The left mainstem bronchus is approximately twice as long as the right and narrower in diameter. It arises above the pulmonary artery, then passes below the aorta and pulmonary artery, and branches into hyparterial bronchi to the superior and inferior lobes.
In “complete” situs inversus, there is mirror-image reversal of this usual arrangement. The prevalence of situs inversus appears to be in a range between 1/25,000 and 1/8,000.
Other structural malformations are not common in most individuals with total situs inversus, but they may occur slightly more frequently than in the average population with situs solitus. For example, 3–5% are said to have congenital heart disease [Schmutzer and Linde, 1958; Tonkin, 1984]. Twenty to 25% of patients with complete, mirror-image situs inversus have ciliary dyskinesia and respiratory symptoms as associated findings. This complex, known as Kartagener syndrome, is discussed below.
PCD/Kartagener
One fourth to one fifth of individuals with complete, mirror-image situs inversus have Kartagener syndrome. The classic Kartagener syndrome triad of clinical features includes situs inversus, bronchiectasis, and chronic sinusitis. Primary ciliary dyskinesia (PCD) or immotile cilia syndrome describes a causally heterogeneous group of autosomal recessive disorders characterized by partial or complete deficiency of ciliary motility associated with a variety of ultrastructural abnormalities such as absent dynein arms, absent radial spokes, and disturbed ciliary orientation [Rott, 1979; Afzelius, 1981; Tonkin, 1984; Afzelius and Mossberg, 1995; Tkebuchava et al., 1996; Jorissen et al., 1997; Marszalek et al., 1997; Teknos et al., 1997]. The characteristic phenotype includes bronchiectasis with chronic cough, rhinitis, sinusitis, male infertility caused by sperm immotility, and variable impairment of female fertility. Complete, mirror-image situs inversus is present in only one half of these patients [Moreno and Murphy, 1981]. Even a pair of monozygotic twins with PCD has been found to be discordant for situs inversus, suggesting that it is truly a random process [Noone et al., 1999].
Because approximately 20–25% of individuals with situs inversus have symptoms suggestive of ciliary dysfunction, the incidence of Kartagener syndrome appears to be approximately 1/120,000 to 1/40,000 [Afzelius and Mossberg, 1995]. Therefore, the overall prevalence of immotile cilia syndromes or PCD may be in the range of 1/60,000 to as high as 1/20,000, because only half of these individuals have situs inversus. PCD occurs in all human races [Rott, 1979].
Recent mapping studies suggest extensive locus heterogeneity in the European and North American population, with potentially interesting regions on 3p, 4q, 5p, 7p, 8q, 10p, 11q, 13q, 15q, 16p, 17q, and 19q [Blouin et al., 2000]. Most suggestive evidence for linkage occurred on 8q, 16pter, 19q. In an Arab population, significant linkage has been found for a locus on 19q [Meeks et al., 2000].
A human PCD gene has been identified using a candidate-gene approach. A human sequence (DNAI1) related to a Chlamydomonas gene necessary for outer dynein arms was isolated, and one patient was identified with two loss-of-function mutations [Pennarun et al., 1999]. That patient had PCD with immotile respiratory cilia that lacked outer dynein arms. Subsequently, two more families have been found with PCD and DNAI1 mutations [Zariwala et al., 2000].
Because a large number of axonemal dynein proteins are involved in the structure and function of cilia, they are obvious candidate genes of great research interest for these causally heterogeneous PCD disorders. For example, in a consanguineous Lebanese family with ciliary akinesia, situs inversus, and absent outer dynein arms by electron microscopy, homozygosity mapping has identified significant linkage on chr 5p15-p14 [Omran et al., 2000]. An excellent candidate gene in that region of linkage is DNAH5, which has high homology to the axonemal Chlamydomonas gamma-heavy dynein chain.
SITUS AMBIGUUS
Some patients have what appears to be a combination of situs solitus and situs inversus. Some organs are in their usual position and some are reversed or duplicated. These individuals usually have major malformations involving one or more of the asymmetric organs, especially defects of the heart and great vessels, anomalies of the liver and biliary tract, and malrotation of the bowel. Patients in this group usually have either congenital asplenia or polysplenia.
Ivemark described the association of asplenia with cardiovascular malformations [Ivemark, 1955]. Asplenia has been called bilateral right-sidedness (dextro-isomerism) because of the apparent bilateral duplication of right-sided structures such as bilateral trilobed lungs, in association with absent spleen. Polysplenia has been called bilateral left-sidedness because of the apparent bilateral duplication of left-sided structures such as bilateral bilobed lungs in association with multiple spleens [Moller et al., 1967].
Historically, asplenia and polysplenia (and their accompanying anomalies) were originally treated as separate entities. The minimal incidence of polysplenia and asplenia was estimated by Rose and colleagues to be 1/40,000 live births [Rose et al., 1975]. Gatrad and colleagues found 1/24,000 affected in an English population and 1/2,700 in a highly inbred Asian population [Gatrad et al., 1984]. Asplenia is more commonly reported than is polysplenia. There seems to be a male predominance in asplenia and an equal sex distribution among cases with polysplenia [Putschar and Manion, 1956; Van Meirop et al., 1972; Rose et al., 1975; Mishalany et al., 1982; Tonkin, 1984].
Because polysplenia and asplenia are both associated with similar malformations in other organ systems and occasional families include cases of both, polysplenia and asplenia are now thought of as different manifestations of a common set of morphogenetic errors [Polhemus and Schafer, 1952; Chen and Monteleone, 1977; Zlotogora and Elian, 1981; Niikawa et al., 1983; Gatrad et al., 1984]. The term “polyasplenia” was suggested by Opitz to recognize the likelihood that asplenia and polysplenia are part of a single spectrum of malformation [Opitz, 1985].
In polyasplenia, there is usually an unusual spatial arrangement of the thoracic and/or abdominal organs in relationship to each other called visceral heterotaxia or heterotaxy, as well as malformations in one or more organ systems. Note that polyasplenia with associated anomalies is sometimes incorrectly referred to, especially in older literature, as “situs inversus.” The latter term should be reserved for situations where there is mirror-image reversal of organ relationships, i.e., so-called “complete” situs inversus. The terms “partial” or “incomplete” situs inversus have also been used for polyasplenia, but “situs ambiguus” seems to be less confusing and is preferred by most.
Malformations Associated With Polyasplenia
Patients with asplenia or polysplenia usually have other, severe malformations that are life threatening. All thoracic and abdominal organ systems may be affected. The list of associated malformations is extensive and involves virtually every thoracic and abdominal organ system [Aylsworth, 1993; Splitt et al., 1996]. As many as 90% of patients with polysplenia and 99% of patients with asplenia are reported to have congenital heart disease, but because many patients are ascertained because of major malformations such as cardiac anomalies, prevalence data for these associated anomalies may be biased. In addition to the usual spectrum of thoracic and visceral malformations, there is also good evidence that central nervous system anomalies and defects of the axial skeleton are nonrandomly associated with the polyasplenia spectrum.
Abdominal heterotaxy in the asplenia phenotype includes bowel malrotation, a symmetric liver, and right-sided stomach. Extracardiac malformations are common and frequently involve the gastrointestinal, genitourinary, bronchopulmonary, axial skeletal, and central nervous systems [Putschar and Manion, 1956; Freedom, 1972]. Cardiovascular malformations are usually conotruncal and tend to be more severe than those associated with the polysplenia phenotype [Polhemus and Schafer, 1952; Ivemark, 1955; Putschar and Manion, 1956; Ruttenberg et al., 1964; Campbell and Deuchar, 1967; Van Meirop et al., 1972; Freedom, 1974; Rose et al., 1975; Majeski and Upshur, 1978; Tonkin, 1984; Winer-Muram and Tonkin, 1989]. The inferior vena cava and the abdominal aorta usually have an anomalous relationship, both lying on the same side of the spine, regardless of the side occupied by the vena cava. This relationship was thought to be pathognomonic for asplenia until it was observed in a patient with polysplenia [Freedom, 1974].
Patients with the polysplenia phenotype also have bowel malrotation and a liver that is symmetrical or inverted with the larger lobe on the left. The stomach is usually right sided. Two or more spleens are frequently present along the greater curvature of the stomach. Cardiovascular malformations most commonly include malposition, atrial septal defects, ventricular septal defects, bilateral superior vena cavae, partial anomalous pulmonary venous return, and intrahepatic interruption of the inferior vena cava with connection to the azygous or hemiazygous vein [Moller et al., 1967; Van Meirop et al., 1972; Rose et al., 1975; Hallett et al., 1979; Peoples et al., 1983; Winer-Muram and Tonkin, 1989]. Extracardiac malformations are common as in asplenia and also involve the gastrointestinal, genitourinary, bronchopulmonary, axial skeletal, and central nervous systems.
Extrahepatic biliary atresia (EHBA) is uniquely associated with polysplenia, and it has been estimated that 10–20% of patients with EHBA have associated malformations like those seen in polysplenia [Chandra, 1974; Maksem, 1980; Falchetti et al., 1991; Silveira et al., 1991; Davenport et al., 1993; Vazquez et al., 1995; Kataria et al., 1996; Nakada et al., 1997; Tanano et al., 1999]. Interestingly, the inv mouse model for situs inversus has extrahepatic biliary obstruction, similar to that seen associated with polysplenia [Mazziotti et al., 1999].
Asplenia rarely is reported to occur without other severe malformations in individuals with situs solitus or complete situs inversus [Putschar and Manion, 1956; Muir, 1969; Waldman et al., 1977; Hauser and Silberman, 1982]. Some cases are familial involving sibs [Gillis et al., 1992] and parent–child pairs [Lindor et al., 1995]. One of the father–son pairs described by Lindor and colleagues included a mild form of cardiac atrial isomerism in one child, suggesting that isolated asplenia may represent the mild end of the polyasplenia spectrum.
Asymptomatic polysplenia with heterotaxia but without severe or complex cardiac or visceral malformation also occurs [Peoples et al., 1983; Gayer et al., 1999]. Note that one or more small, accessory spleens may occasionally be found in individuals with situs solitus. They are usually asymptomatic and not considered to be caused by defects in the determination of laterality, but may be associated with other malformations such as splenogonadal fusion and limb reduction.
FAMILIAL CASES AND POSSIBLE CAUSES OF LATERALITY DEFECTS
Polyasplenia usually occurs sporadically, but occasional families shed light on possible genetic mechanisms responsible for laterality determination. Familial recurrences of situs inversus and situs ambiguus have suggested all Mendelian modes of inheritance. In addition, a number of teratogens are causally associated with laterality defects in humans and experimental animal models. Some examples of these follow.
Mendelian Factors
Observations of families with affected sibs led to the suggestion of autosomal recessive causation [Polhemus and Schafer, 1952; Chen and Monteleone, 1977; Zlotogora and Elian, 1981; Toriello et al., 1986]. Even better evidence for autosomal recessive inheritance comes from reports of affected sibs who have consanguineous parents [Arnold et al., 1983; Gatrad et al., 1984; Czeizel, 1987]. The recessive nature of situs inversus associated with ciliary dyskinesia is discussed elsewhere.
Situs ambiguus and complete, mirror-image situs inversus (thoracic and/or abdominal, with or without ciliary abnormality) may be parts of the same spectrum of malformation-association. One boy and two of his cousins had typical Kartagener syndrome with abnormal cilia and situs inversus. His sister, who also displayed respiratory symptoms associated with abnormal cilia, had features of situs ambiguus including pulmonic stenosis, absence of the inferior vena cava with azygous continuation, midline liver, and two contiguous splenic masses, suggesting that at least one of her predisposing factors was a ciliary dyskinesia gene [Schidlow et al., 1982].
An interesting family suggesting autosomal dominant inheritance, or at least a vertically transmitted susceptibility gene, contained four individuals from three generations with laterality defects. Two individuals had complete situs inversus, and two had situs ambiguus (asplenia, midline liver, and complex cardiac malformation) [Casey et al., 1996]. Two obligate carriers were anatomically normal. Several other reports also suggest the existence of either straightforward autosomal dominant inheritance of situs inversus and situs ambiguus, or at least a monogenic predisposition to laterality defects running through families [Niikawa et al., 1983; Gershoni-Baruch et al., 1989; Alonso et al., 1995].
As mentioned above, individuals with mirror-image situs inversus usually have a low incidence of structural malformations, but occasional families have a high frequency of other anomalies, in a manner reminiscent of the iv mouse (v.i.) [Zlotogora et al., 1987]. Some families have had recurrence of the malformations that are typically associated with situs ambiguus, such as congenital heart disease and polyasplenia, with variable occurrence of complete situs inversus or other features of situs ambiguus [Silver et al., 1972; Katcher, 1980; Arnold et al., 1983; Distefano et al., 1987; Zlotogora et al., 1987; Mikkila et al., 1994; Alonso et al., 1995].
These observations are of particular interest because they suggest that isolated birth defects such as congenital heart disease, anal atresia, diaphragmatic hernia, bicornuate uterus, etc. may be caused by predisposing genes that are involved in left–right axis determination pathways. In fact, one such gene has been identified. The zebrafish oep gene is involved in left–right axis determination [Yost, 1998a, b] and the mouse homolog, Cryptic, is also important for laterality determination [Gaio et al., 1999; Yan et al., 1999]. Recently, three missense and one frameshift mutation have been found in the human CRYPTIC gene in four patients with situs inversus and five with situs ambiguus [Bamford et al., 2000b]. Subsequent studies have identified two of these mutations, along with one other new mutation, in patients who have only transposition of the great arteries, i.e., isolated congenital heart disease without any other sign of abnormal situs determination [Bamford et al., 2000a]. Therefore, as predicted, at least some patients with isolated, nonsyndromic conotruncal heart defects have their malformations caused by mutations in a gene involved in left–right axis determination. They do not happen to have diagnostic features of situs ambiguus such as polyasplenia or heterotaxy, perhaps by chance or because of the effects of other genes or environmental factors. We predict that other isolated, nonsyndromic malformations will turn out to be caused by similar mechanisms of mutation involving genes in pathways of situs determination.
In 1987, Mathias et al. reported a family in which nine males in two generations were affected by a spectrum of abnormalities of laterality, including congenital heart disease, situs inversus, or polyasplenia and an X-linked recessive inheritance pattern. All 11 affected relatives had some form of situs inversus or situs ambiguus. Both asplenia and polysplenia occurred, with cardiac, central nervous system, and vertebral malformations, including sacral dysplasia with anorectal anomalies [Mathias et al., 1987].
Another X-linked family has the unique finding of situs inversus in carrier females and situs ambiguus in males [Gebbia et al., 1997]. The affected males had lethal malformation syndromes associated with situs ambiguus, whereas carrier females had mirror-image situs inversus or situs solitus. Anal and sacral anomalies occur in both affected males and carrier females.
The X-linked family described by Mikkila et al. [1994] also features caudal anomalies in the affected males and genitourinary anomalies in carrier females (bicornuate uterus, uterine septum, septate vagina and uterus), and one had anal stenosis.
Several other cases of situs ambiguus with caudal dysgenesis have been described [Peoples et al., 1983; Fullana et al., 1986; Rodriguez et al., 1991]. We have studied a pair of brothers with situs ambiguus and complex congenital heart disease, one of whom had sacral dysplasia and imperforate anus. It seems likely that most isolated cases of situs inversus or situs ambiguus with caudal dysplasia and/or anal anomalies may be caused by X-linked causes of laterality defects, because familial recurrences of this association are reported only in families compatible with X-linked inheritance.
The causes of this phenotype of X-linked situs ambiguus, with variable manifestations of caudal dysplasia in both hemizygous males and heterozygous females, and variable expression of complete, mirror-image situs inversus in heterozygous females, have been shown to be various mutations in the X-linked gene, ZIC3 [Ferrero et al., 1997; Gebbia et al., 1997]. Zic3 is also the gene deleted in the X-linked mouse mutation Bent Tail, which causes kinked tails because of aberrant tail bone development, exencephaly in some, and situs ambiguus in some of both the hemizygous males and heterozygous females [Carrel et al., 2000; Klootwijk et al., 2000] The Zic3 knockout mouse has a phenotype similar to the human ZIC3 mutations, including situs ambiguus, central nervous system malformations (exencephaly), and axial skeletal anomalies (vertebral dysplasia, fusion, and duplication) [Purandare et al., 2000; Casey, 2001].
Chromosome Abnormalities
Patients reported with situs inversus or situs ambiguus and various chromosome abnormalities are listed in Table I. Chromosome rearrangements can cause overexpression of duplicated genes, deficiency of deleted gene products, or disruption of single genes. Therefore, patients with laterality defects and chromosomal abnormalities may be useful in mapping genes involved in left–right axis determination. Conversely, it should be kept in mind that associations seen in only a single patient may be coincidental.
|
Phenotype |
Chromosome abnormality |
Reference |
|---|---|---|
|
Situs ambiguus |
Partial trisomy 3p(mat); maternal translocation t(3;4)(p23;q35). |
[Schinzel et al., 1978] |
|
Situs inversus abdominus, complex congenital heart disease |
dup(2)(pterp24) and del(2)(q34qter) |
[Schinzel, 1983] |
|
1) Agnathia, holoprosencephaly, situs solitus |
dup(6)(pterp24) and del(18)(pterp11.21) |
[Krassikoff and Sekhon, 1989] |
|
2) Similarly affected sister had apparent situs solitus but also cardiac malformations, renal hypoplasia, and malrotation |
||
|
Situs ambiguus, asplenia Mother phenotypically normal |
46,XX,t(12;13)(q13.1;p13)(mat)—balanced translocation, inherited from mother |
[Wilson et al., 1991] |
|
1) Situs ambiguus, omphalocele, cleft lip & palate |
46,XX,del(10)(q21q23) |
[Carmi R. et al., 1992] |
|
2) Situs ambiguus, microcephaly, absent thumbs |
46,XX,del(13)(q31qter) |
|
|
Situs ambiguus, polysplenia, and bilateral split hand malformation |
46,XY,ins(7;8)(q22;q12q24); insertion of 8q12q24 into 7q22 |
[Koiffmann et al., 1993] |
|
Situs ambiguus and familial split hand/foot malformation |
Familial, apparently balanced, reciprocal t(2;7)(q21.1;q22.1) (baby with situs ambiguus not karyotyped) |
|
|
Asplenia, pulmonic stenosis, Hirschsprung disease, minor anomalies, and mental retardation |
46,XX,t(11;20)(q13.1;q13.13)pat—balanced 11;20 translocation |
[Freeman et al., 1996] |
|
Left arm amelia, congenital short bowel, malrotation, pseudoobstruction, dextrocardia with situs solitus, patent ductus arteriosus, and a tiny atrophic spleen |
MOS 46,XX/46,XX,r(4)(p16q22.3)/47,XX,del(4)(q22.3),+r(4)(p16q22.3) |
[Hou and Wang, 1996] |
|
Inversely located heart, stomach, duodenum, and cecum; cerebral atrophy, hypertelorism, severe mental retardation |
Apparently balanced translocation: 46,XX,t(6;18)(q21;q21.3) |
[Kato et al., 1996] |
|
1) Dextrocardia |
22q11 deletions |
[Splitt et al., 1996] |
|
2) Polysplenia |
||
|
Polysplenia, hydrocephalus, intrauterine growth retardation, single umbilical artery, agenesis of the corpus callosum |
Confined placental mosaicism 47,XXX,+16 |
[Sanchez et al., 1997] |
|
Complete situs inversus, cystic fibrosis, normal cilia by electron microscopy |
Paternal isodisomy of chromosome 7 (see iv mouse) |
[Pan et al., 1998] |
|
1) Situs inversus, malrotation, duodenal and jejunal atresia, short stature, mental retardation, seizures |
Microdeletion of chromosome subband 2q37.3 |
[Reddy et al., 1999] |
|
2) Adult (unrelated to case 1) with mild mental retardation, heterotaxy, malrotation and malposition of large and small bowels, with most of the bowels above the liver and spleen, malrotation and malposition of the right kidney, hypoplastic male genitalia |
Microdeletion of chromosome subband 2q37.3 |
|
|
Situs ambiguus & other features of del 18p phenotype |
del(18)(p11.21) |
[Digilio et al., 2000] |
|
Situs ambiguus, polysplenia. Father is phenotypically normal |
inv(11)(q13.5;q25)(pat)—11q13.5 breakpoint interrupts the UVRAG gene (complements UV sensitivity of XP group C) |
[Iida et al., 2000] |
- a EM, electron microscopy.
Teratogens
Maternal diabetes
Maternal diabetes causes fetal anomalies of the neural tube, cardiovascular system, and many other structures. In Kucera's study of maternal diabetes, so-called situs inversus was the anomaly with the second-highest increased incidence in women with diabetes compared to controls (second only to defects of the spine) [Kucera, 1971]! Because these observations involved malformed babies of mothers with diabetes, it seems likely that most or all probably had situs ambiguus. Subsequent reports confirm the association of the polyasplenia complex with maternal diabetes [Gonzalez et al., 1989; Slavotinek et al., 1996]. Reported anomalies include situs inversus or ambiguus, sacral dysgenesis, neural tube defects, congenital heart malformation, arthrogryposis, and an extremely wide and variable spectrum of malformation involving the genitourinary tract, gastrointestinal tract, nervous system, craniofacies, and skeleton.
In the NOD mouse, a model of insulin-dependent diabetes mellitus, maternal diabetes is clearly the cause of laterality defects in the offspring [Morishima et al., 1991]. Fetuses have anomalies of the viscera, and the incidence is related to maternal, clinically manifested diabetes. Morishima and colleagues studied the role of gene-environment interaction in malformations in fetuses of NOD mothers [Morishima et al., 1996]. NOD dams were mated with NOD males, ICR males (the original strain from which NOD was derived), and with C57BL/6J males, which are not genetically related to the NOD. The frequency of visceroatrial heterotaxy in fetuses from diabetic dams was 65% in NODxNOD crosses (dam X sire, respectively), 24% in NODxICR crosses, and 7% in NODxC57BL/6J crosses. The cases with heterotaxy showed a tendency toward asplenia and had severe cardiac defects such as AV canal, double-outlet right ventricle, and transposition of the great vessels. These findings clearly demonstrate that the induction of polyasplenia with heterotaxy and malformation by maternal diabetes is influenced by the fetal genotype. The fetal genotype appears to set a level of predisposition to malformation, whereas the abnormal glucose homeostasis in the environment disrupts normal development in susceptible embryos.
Retinoic acid
Prenatal exposure to retinoic acid produces malformations in any organ system in hamsters, depending on the time of treatment [Shenefelt, 1972]. Microphthalmia and “situs inversus” are produced by treatment at the earliest times that cause anomalies, stages that are also those of peak embryo sensitivity. Both a deficit and an excess of this molecule can produce cardiac defects, which depend on dose and timing. One mechanism proposed is that retinoic acid causes these cardiac defects by disrupting production of the extracellular matrix [Sinning, 1998].
Retinoic acid acts in a synergistic manner with the type IIB activin receptor [Oh and Li, 1997]. Acvr2b knockout mice die shortly after birth with asplenia, complicated cardiac malformations, renal anomalies, and thoracic hypersegmentation. The human gene, ACVR2B, has been characterized and three missense mutations have been identified in patients with situs ambiguus by screening samples from 112 sporadic and 14 familial cases of laterality defects [Kosaki et al., 1999].
OTHER SELECTED PHENOTYPES OF INTEREST
Renal–Hepatic–Pancreatic Dysplasia and Skeletal Dysplasia
Ivemark, who described the syndrome of asplenia, also described a familial condition characterized by renal–hepatic–pancreatic dysplasia (RHPD) [Ivemark et al., 1959]. This Ivemark RHPD phenotype was subsequently observed to occur in association with situs ambiguus, and there is evidence that at least some RHPD and RHPD with situs ambiguus is autosomal recessive [Crawfurd, 1978; Lurie et al., 1991; Torra et al., 1996].
A lethal syndrome of cystic renal dysplasia, pancreatic cysts with interstitial fibrosis, intrahepatic biliary dysgenesis, and total situs inversus was described in two unrelated patients who were born 8 years apart in the same remote Japanese town [Yoshikawa et al., 1981; Hiraoka et al., 1988]. Another three sibs with situs inversus totalis and severe cystic dysplasia of the kidneys and pancreas have been subsequently described, but they had no liver disease. They also had bowing of the lower limbs and clavicles, and intrauterine growth retardation [Balci et al., 1999, 2000]. These patients seem to have a syndrome similar to the RHPD described above, but with complete situs inversus instead of polyasplenia. Other reports of patients with RHPD associated with situs inversus include one of the patients described by Bernstein in his review of RHPD [Bernstein et al., 1987].
Spranger described a patient with situs inversus and renal and pancreatic dysplasia associated with the Saldino-Noonan type of short rib–polydactyly syndrome [Spranger et al., 1974]. A patient, reported by Fraser, had situs inversus, typical radiographic features of chondroectodermal dysplasia, and a number of other anomalies including cleft epiglottis and larynx, skin tags on the neck, postaxial hexadactyly of hands and feet, pancreatic cystic dysplasia, renal dysplasia, ambiguous genitalia with micropenis, and imperforate anus [Fraser et al., 1989]. A patient described by Brueton and colleagues had skeletal features of Jeune asphyxiating thoracic dystrophy with situs inversus, and RHPD [Brueton et al., 1990]. Brueton and colleagues also pointed out that older patients with Jeune syndrome commonly have some form of RHPD. In that same article, Brueton et al. described another patient with skeletal features of chondroectodermal dysplasia along with situs inversus but no RHPD, very much like a patient reported by Donlan et al. [1969]. Several other patients have been reported with short rib-polydactyly syndromes and situs inversus or situs ambiguus [Belloni and Beluffi, 1981; Bernstein et al., 1985; de Sierra et al., 1992; Tsai et al., 1992; Urioste et al., 1994; Digilio et al., 1999]. At least one patient with Jeune syndrome and situs abnormalities has been observed [Majewski et al., 1996]. The association of Jeune syndrome with RHPD-like complications is well known in those patients who survive through later childhood.
An analysis of the congenital heart defects seen in “oral–facial–skeletal syndromes” (short rib–polydactyly syndromes and other possibly related oral–facial–skeletal syndromes) showed a pathogenetic similarity to the types of heart defects seen in the polyasplenia spectrum [Digilio et al., 1999]. These overlapping phenotypes suggest an interesting community of syndromes (Fig. 1). Although most patients with SRP or RHPD do not have associated laterality defects, these phenotype associations appear to be noncoincidental, and may provide clues about underlying causes (i.e., genes and/or environmental factors), or common pathogenetic sequences (i.e., interactions between developmental pathways).
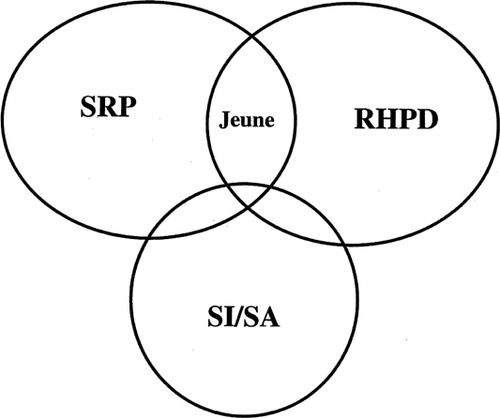
Overlapping phenotypes with situs abnormalities (SI/SA), short rib–polydactyly syndromes (SRP), and renal–hepatic–pancreatic dysplasia (RHPD). Although laterality defects are not typical of most patients with either SRP or RHPD, the patients with combinations of these disorders would suggest that these phenotypes are pathogenetically related at some level. Note that most patients with Jeune syndrome would fall in the large area of overlap between SRP and RHPD. SRP includes short rib–polydactyly syndromes, Jeune syndrome, and chondroectodermal dysplasia.
Situs Ambiguus With Campomelia, RHPD, and Cervical Lymphocele
Campomelia, cervical lymphocele, polycystic kidneys, pancreas, and liver, short gut, and polysplenia have been described in sibs [Cumming et al., 1986] and in another unrelated patient [Ming et al., 1997]. Skeletal, lymphatic, and renal lesions were seen at 26 weeks' gestation by ultrasonography, but not at 16 weeks. Sibs with campomelia, polycystic dysplasia, and cervical lymphocele but without polysplenia have also been described [Urioste et al., 1991].
Agnathia–Holoprosencephaly
A lethal malformation complex of agnathia with associated anomalies has been reported in a number of patients, 10 of whom have had situs inversus and/or situs ambiguus [Pauli et al., 1981; Leech et al., 1988; Hersh et al., 1989; Robinson and Lenke, 1989; Meinecke et al., 1990; Persutte et al., 1990; Stoler and Holmes, 1992; Ozden et al., 2000]. Other malformations reported to be associated have involved the genitourinary, gastrointestinal, and central nervous system. Five of the patients with agnathia and situs abnormality have had holoprosencephaly. Three of these had situs inversus totalis and two had situs ambiguus with polysplenia. Other patients with a similar but less severe pattern of malformation are those with micrognathia and aglossia/hypoglossia, and several have had situs inversus [Hussels, 1971; Dunham and Austin, 1990]. Finally, two sibs, a brother and sister of consanguineous parents, were reported to have situs ambiguus with associated meningomyelocele in the female and holoprosencephaly in the male [Bonneau et al., 1999]. These overlapping phenotypes suggest another interesting potential community of syndromes (Fig. 2). Although most patients with agnathia or holoprosencephaly do not have associated laterality defects, these associations appear to be noncoincidental, and, like the SRP-RHPD group, their analysis should provide clues about common underlying causes or pathogenetic sequences.
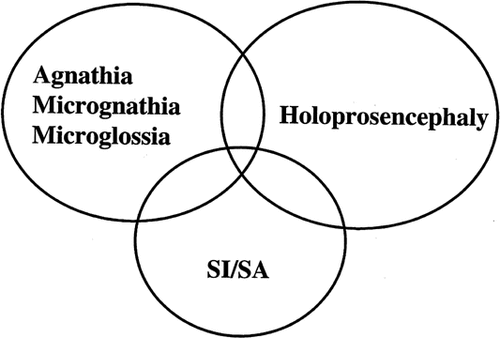
Overlapping phenotypes with agnathia/micrognathia/microglossia, holoprosencephaly, and situs abnormalities (SI/SA).
The iv Mouse Model
Approximately half of mice homozygous for the iv (situs inversus) mutation have situs inversus [Hummel and Chapman, 1959; Layton, 1976]. Although this mouse model is frequently thought of as a model for complete, mirror-image situs inversus, it should be noted that as many as 40% of the homozygotes also have some type of heterotaxy or visceral malformation. Homozygous iv mice have a variable but frequent occurrence of malformation, most notably of the cardiovascular system but also involving lung lobation, liver location, bowel rotation, and spleen shape, size, presence, and location. Some early embryos have markedly distorted bulboventricular loops. Layton proposed that the heterotaxia and associated malformations can all be explained by defective control of the shape of the cytoskeleton and cytomusculature, which appears to be important in the early formation of the cardiac loop [Layton, 1978]. The iv mouse model seems more like some of the rare families reported that include both situs inversus and situs ambiguus [Zlotogora et al., 1987] than it does like pure situs inversus without heterotaxy or malformation.
The iv-/- phenotype is not associated with respiratory ciliary abnormalities [Handel and Kennedy, 1984]. It is, however, caused by a mutation in a gene coding for an axonemal dynein heavy chain, left/right-dynein (lrd) that appears to be expressed in the monocilia of the node during gastrulation, but not later in respiratory cilia [Supp et al., 1997, 1999; Wagner and Yost, 2000]. The nodal cilia, in turn, appear to be important in initiating normal left–right axis asymmetry determination [Marszalek et al., 1999; Supp et al., 2000].
The mouse gene that codes for lrd, Dnahc11, was originally mapped to mouse chromosome 12 [Hanzlik et al., 1990]. The human gene maps to chromosome 7 [Chapelin et al., 1997; Bartoloni et al., 1999]. Recently, a homozygous R to X nonsense mutation in an exon coding for the motor domain of the human gene, DNAH11, has been identified in a patient with situs inversus [Bartoloni et al., 2000]. The patient also had cystic fibrosis caused by paternal uniparental disomy for chromosome 7, abnormal ciliary motility by direct observation of wet preparations from a bronchial biopsy, but structurally normal cilia by electron microscopy (as is seen in the iv-/- mouse) [Pan et al., 1998]. Bartoloni et al. commented that they do not expect a significant fraction of patients with PCD to have mutations in this gene because their linkage analysis in 50 nuclear families did not show a significant lod score on chromosome 7p.
CONCLUSION
The past few years have seen a rapid increase in knowledge about the molecular basis of early developmental pathways involved in laterality determination. It is important for clinical geneticists and dysmorphologists to continue delineating the various human phenotypes and phenotype associations involving defects in the determination of laterality. Such well-defined phenotypes will be very useful in correlating future genotype discoveries with their phenotypic effects.




